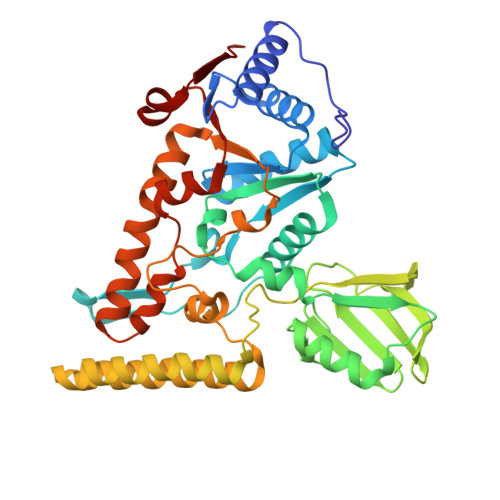
Crucial role and mechanism of transcription-coupled DNA repair in bacteria.
Bharati, B.K., Gowder, M., Zheng, F., Alzoubi, K., Svetlov, V., Kamarthapu, V., Weaver, J.W., Epshtein, V., Vasilyev, N., Shen, L., Zhang, Y., Nudler, E.(2022) Nature 604: 152-159
- PubMed: 35355008
- DOI: https://doi.org/10.1038/s41586-022-04530-6
- Primary Citation of Related Structures:
7EGS, 7EGT - PubMed Abstract:
Transcription-coupled DNA repair (TCR) is presumed to be a minor sub-pathway of nucleotide excision repair (NER) in bacteria. Global genomic repair is thought to perform the bulk of repair independently of transcription. TCR is also believed to be mediated exclusively by Mfd-a DNA translocase of a marginal NER phenotype 1-3 . Here we combined in cellulo cross-linking mass spectrometry with structural, biochemical and genetic approaches to map the interactions within the TCR complex (TCRC) and to determine the actual sequence of events that leads to NER in vivo. We show that RNA polymerase (RNAP) serves as the primary sensor of DNA damage and acts as a platform for the recruitment of NER enzymes. UvrA and UvrD associate with RNAP continuously, forming a surveillance pre-TCRC. In response to DNA damage, pre-TCRC recruits a second UvrD monomer to form a helicase-competent UvrD dimer that promotes backtracking of the TCRC. The weakening of UvrD-RNAP interactions renders cells sensitive to genotoxic stress. TCRC then recruits a second UvrA molecule and UvrB to initiate the repair process. Contrary to the conventional view, we show that TCR accounts for the vast majority of chromosomal repair events; that is, TCR thoroughly dominates over global genomic repair. We also show that TCR is largely independent of Mfd. We propose that Mfd has an indirect role in this process: it participates in removing obstructive RNAPs in front of TCRCs and also in recovering TCRCs from backtracking after repair has been completed.
Organizational Affiliation:
Department of Biochemistry and Molecular Pharmacology, New York University School of Medicine, New York, NY, USA.