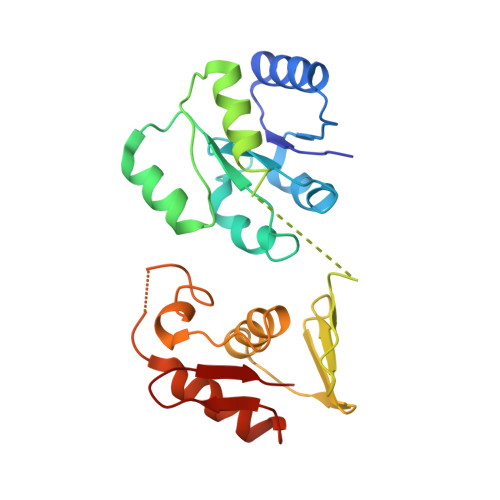
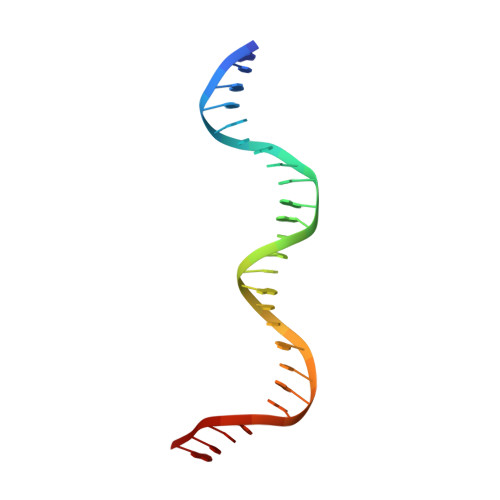
Structural basis of phosphorylation-induced activation of the response regulator VbrR.
Hong, S., Guo, J., Zhang, X., Zhou, X., Zhang, P., Yu, F.(2023) Acta Biochim Biophys Sin (Shanghai)
- PubMed: 36647726
- DOI: https://doi.org/10.3724/abbs.2022200
- Primary Citation of Related Structures:
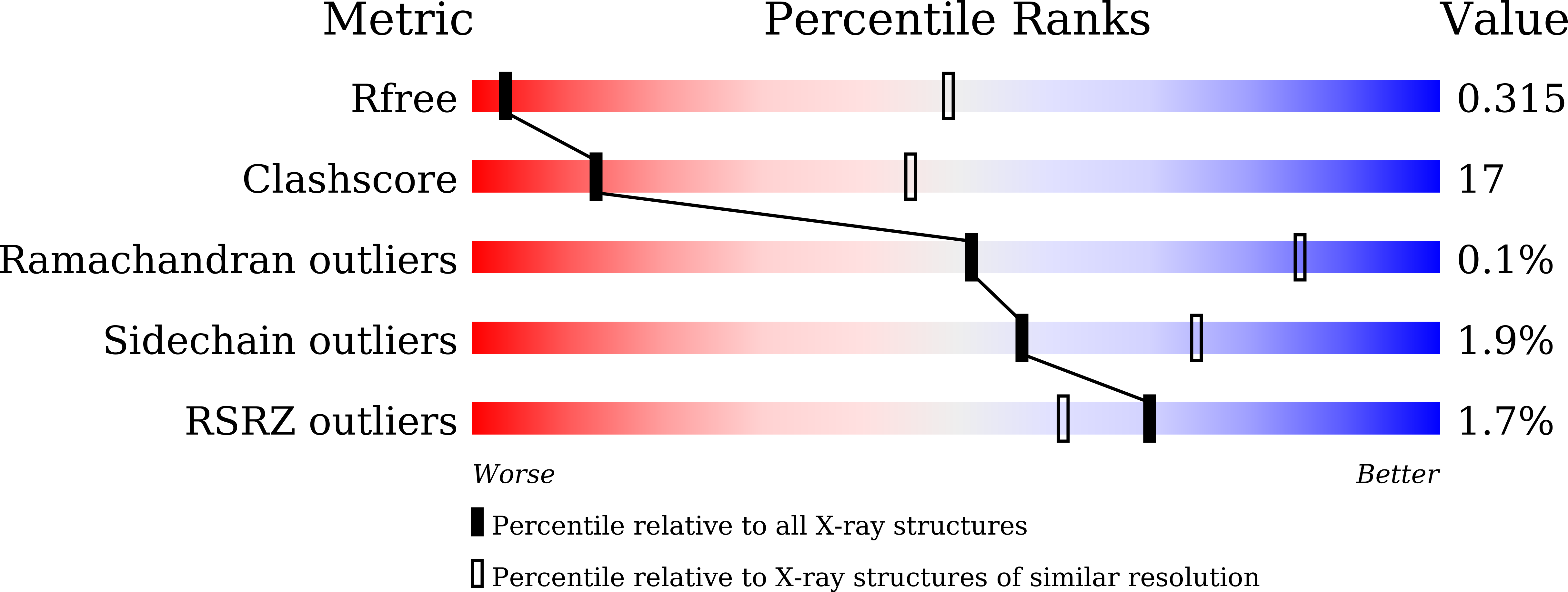
7E1B, 7E1D, 7E1F, 7E1H - PubMed Abstract:
Two-component systems typically consist of a paired histidine kinase and response regulator and couple environmental changes to adaptive responses. The response regulator VbrR from Vibrio parahaemolyticus , a member of the OmpR/PhoB family, regulates virulence and antibiotic resistance genes. The activation mechanism of VbrR remains unclear. Here, we report the crystal structures of full-length VbrR in complex with DNA in the active conformation and the N-terminal receiver domain (RD) and the C-terminal DNA-binding domain (DBD) in both active and inactive conformations. Structural and biochemical analyses suggest that unphosphorylated VbrR adopts mainly as inactive dimers through the DBD at the autoinhibitory state. The RD undergoes a monomer-to-dimer transition upon phosphorylation, which further induces the transition of DBD from an autoinhibitory dimer to an active dimer and enables its binding with target DNA. Our study suggests a new model for phosphorylation-induced activation of response regulators and sheds light on the pathogenesis of V . parahaemolyticus .
Organizational Affiliation:
National Key Laboratory of Plant Molecular Genetics, Center for Excellence in Molecular Plant Sciences, Institute of Plant Physiology and Ecology, Chinese Academy of Sciences, Shanghai 200032, China.