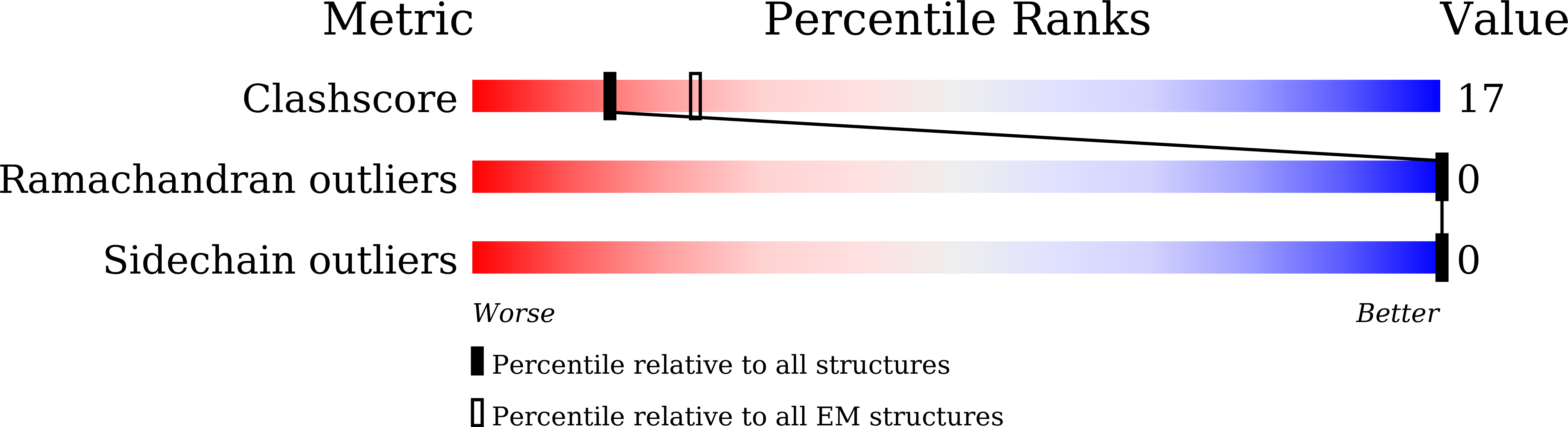Wild-type alpha-synuclein inherits the structure and exacerbated neuropathology of E46K mutant fibril strain by cross-seeding.
Long, H., Zheng, W., Liu, Y., Sun, Y., Zhao, K., Liu, Z., Xia, W., Lv, S., Liu, Z., Li, D., He, K.W., Liu, C.(2021) Proc Natl Acad Sci U S A 118
- PubMed: 33972418
- DOI: https://doi.org/10.1073/pnas.2012435118
- Primary Citation of Related Structures:
7C1D - PubMed Abstract:
Heterozygous point mutations of α-synuclein (α-syn) have been linked to the early onset and rapid progression of familial Parkinson's diseases (fPD). However, the interplay between hereditary mutant and wild-type (WT) α-syn and its role in the exacerbated pathology of α-syn in fPD progression are poorly understood. Here, we find that WT mice inoculated with the human E46K mutant α-syn fibril (hE46K) strain develop early-onset motor deficit and morphologically different α-syn aggregation compared with those inoculated with the human WT fibril (hWT) strain. By using cryo-electron microscopy, we reveal at the near-atomic level that the hE46K strain induces both human and mouse WT α-syn monomers to form the fibril structure of the hE46K strain. Moreover, the induced hWT strain inherits most of the pathological traits of the hE46K strain as well. Our work suggests that the structural and pathological features of mutant strains could be propagated by the WT α-syn in such a way that the mutant pathology would be amplified in fPD.
Organizational Affiliation:
Interdisciplinary Research Center on Biology and Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, Shanghai, 201210, China.