Anthracenyl Functionalization of Half-Sandwich Carbene Complexes: In Vitro Anticancer Activity and Reactions with Biomolecules.
Lee, B.Y.T., Sullivan, M.P., Yano, E., Tong, K.K.H., Hanif, M., Kawakubo-Yasukochi, T., Jamieson, S.M.F., Soehnel, T., Goldstone, D.C., Hartinger, C.G.(2021) Inorg Chem 60: 14636-14644
- PubMed: 34528438
- DOI: https://doi.org/10.1021/acs.inorgchem.1c01675
- Primary Citation of Related Structures:
6WGO - PubMed Abstract:
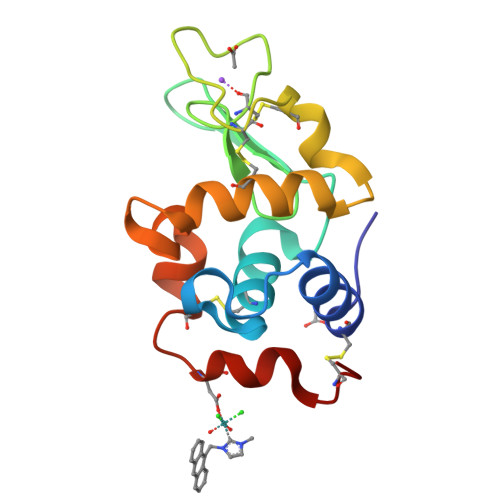
N-Heterocyclic carbene (NHC) ligands are widely investigated in medicinal inorganic chemistry. Here, we report the preparation and characterization of a series of half-sandwich [M(L)(NHC)Cl 2 ] (M = Ru, Os, Rh, Ir; L = cym/Cp*) complexes with a N-flanking anthracenyl moiety attached to imidazole- and benzimidazole-derived NHC ligands. The anticancer activity of the complexes was investigated in cell culture studies where, in comparison to a Rh derivative with an all-carbon-donor-atom-based ligand ( 5a ), they were found to be cytotoxic with IC 50 values in the low micromolar range. The Ru derivative 1a was chosen as a representative for stability studies as well as for biomolecule interaction experiments. It underwent partial chlorido/aqua ligand exchange in DMSO- d 6 /D 2 O to rapidly form an equilibrium in aqueous media. The reactions of 1a with biomolecules proceeded quickly and resulted in the formation of adducts with amino acids, DNA, and protein. Hen egg white lysozyme crystals were soaked with 1a , and the crystallographic analysis revealed an interaction with an l-aspartic acid residue (Asp119), resulting in the cleavage of the p -cymene ligand but the retention of the NHC moiety. Cell morphology studies for the Rh analog 3a suggested that the cytotoxicity is exerted via mechanisms different from that of cisplatin.
Organizational Affiliation:
OBT (Oral Health-Brain Health-Total Health) Research Center, Faculty of Dental Science, Kyushu University, 3-1-1 Maidashi, Higashi-ku, Fukuoka 812-8582, Japan.