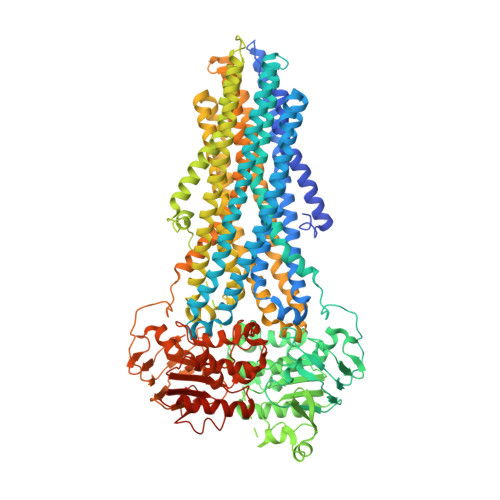
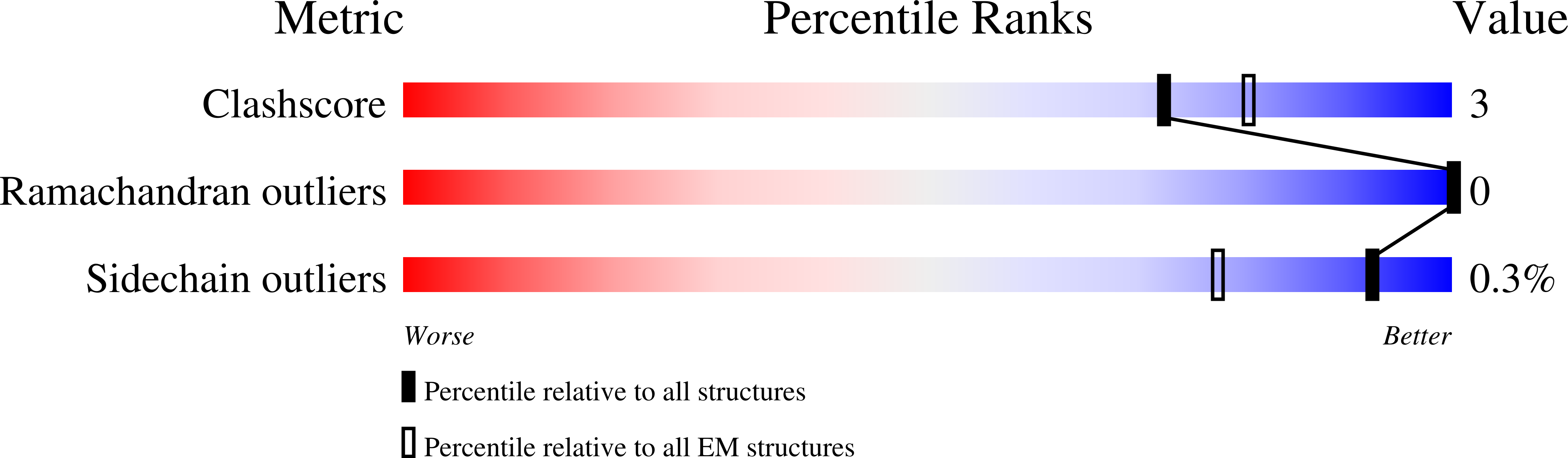Structure of the human lipid exporter ABCB4 in a lipid environment.
Olsen, J.A., Alam, A., Kowal, J., Stieger, B., Locher, K.P.(2020) Nat Struct Mol Biol 27: 62-70
- PubMed: 31873305
- DOI: https://doi.org/10.1038/s41594-019-0354-3
- Primary Citation of Related Structures:
6S7P - PubMed Abstract:
ABCB4 is an ATP-binding cassette transporter that extrudes phosphatidylcholine into the bile canaliculi of the liver. Its dysfunction or inhibition by drugs can cause severe, chronic liver disease or drug-induced liver injury. We determined the cryo-EM structure of nanodisc-reconstituted human ABCB4 trapped in an ATP-bound state at a resolution of 3.2 Å. The nucleotide binding domains form a closed conformation containing two bound ATP molecules, but only one of the ATPase sites contains bound Mg 2+ . The transmembrane domains adopt a collapsed conformation at the level of the lipid bilayer, but we observed a large, hydrophilic and fully occluded cavity at the level of the cytoplasmic membrane boundary, with no ligand bound. This indicates a state following substrate release but prior to ATP hydrolysis. Our results rationalize disease-causing mutations in human ABCB4 and suggest an 'alternating access' mechanism of lipid extrusion, distinct from the 'credit card swipe' model of other lipid transporters.
Organizational Affiliation:
Department of Biology, ETH Zürich, Zürich, Switzerland.