Two new ene-reductases from photosynthetic extremophiles enlarge the panel of old yellow enzymes: CtOYE and GsOYE.
Robescu, M.S., Niero, M., Hall, M., Cendron, L., Bergantino, E.(2020) Appl Microbiol Biotechnol 104: 2051-2066
- PubMed: 31930452
- DOI: https://doi.org/10.1007/s00253-019-10287-2
- Primary Citation of Related Structures:
6S0G, 6S23, 6S31, 6S32 - PubMed Abstract:
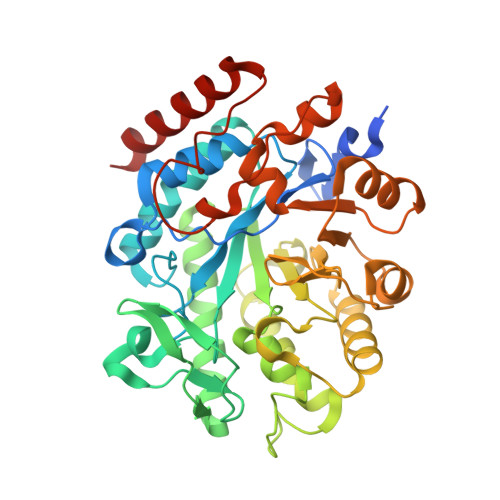
Looking for new ene-reductases with uncovered features beneficial for biotechnological applications, by mining genomes of photosynthetic extremophile organisms, we identified two new Old Yellow Enzyme homologues: CtOYE, deriving from the cyanobacterium Chroococcidiopsis thermalis, and GsOYE, from the alga Galdieria sulphuraria. Both enzymes were produced and purified with very good yields and displayed catalytic activity on a broad substrate spectrum by reducing α,β-unsaturated ketones, aldehydes, maleimides and nitroalkenes with good to excellent stereoselectivity. Both enzymes prefer NADPH but demonstrate a good acceptance of NADH as cofactor. CtOYE and GsOYE represent robust biocatalysts showing high thermostability, a wide range of pH optimum and good co-solvent tolerance. High resolution X-ray crystal structures of both enzymes have been determined, revealing conserved features of the classical OYE subfamily as well as unique properties, such as a very long loop entering the active site or an additional C-terminal alpha helix in GsOYE. Not surprisingly, the active site of CtOYE and GsOYE structures revealed high affinity toward anions caught from the mother liquor and trapped in the anion hole where electron-withdrawing groups such as carbonyl group are engaged. Ligands (para-hydroxybenzaldehyde and 2-methyl-cyclopenten-1-one) added on purpose to study complexes of GsOYE were detected in the enzyme catalytic cavity, stacking on top of the FMN cofactor, and support the key role of conserved residues and FMN cofactor in the catalysis.
Organizational Affiliation:
Department of Biology, University of Padova, Viale G. Colombo 3, 35131, Padova, Italy.