New pyrido[3,4-g]quinazoline derivatives as CLK1 and DYRK1A inhibitors: synthesis, biological evaluation and binding mode analysis.
Tazarki, H., Zeinyeh, W., Esvan, Y.J., Knapp, S., Chatterjee, D., Schroder, M., Joerger, A.C., Khiari, J., Josselin, B., Baratte, B., Bach, S., Ruchaud, S., Anizon, F., Giraud, F., Moreau, P.(2019) Eur J Med Chem 166: 304-317
- PubMed: 30731399
- DOI: https://doi.org/10.1016/j.ejmech.2019.01.052
- Primary Citation of Related Structures:
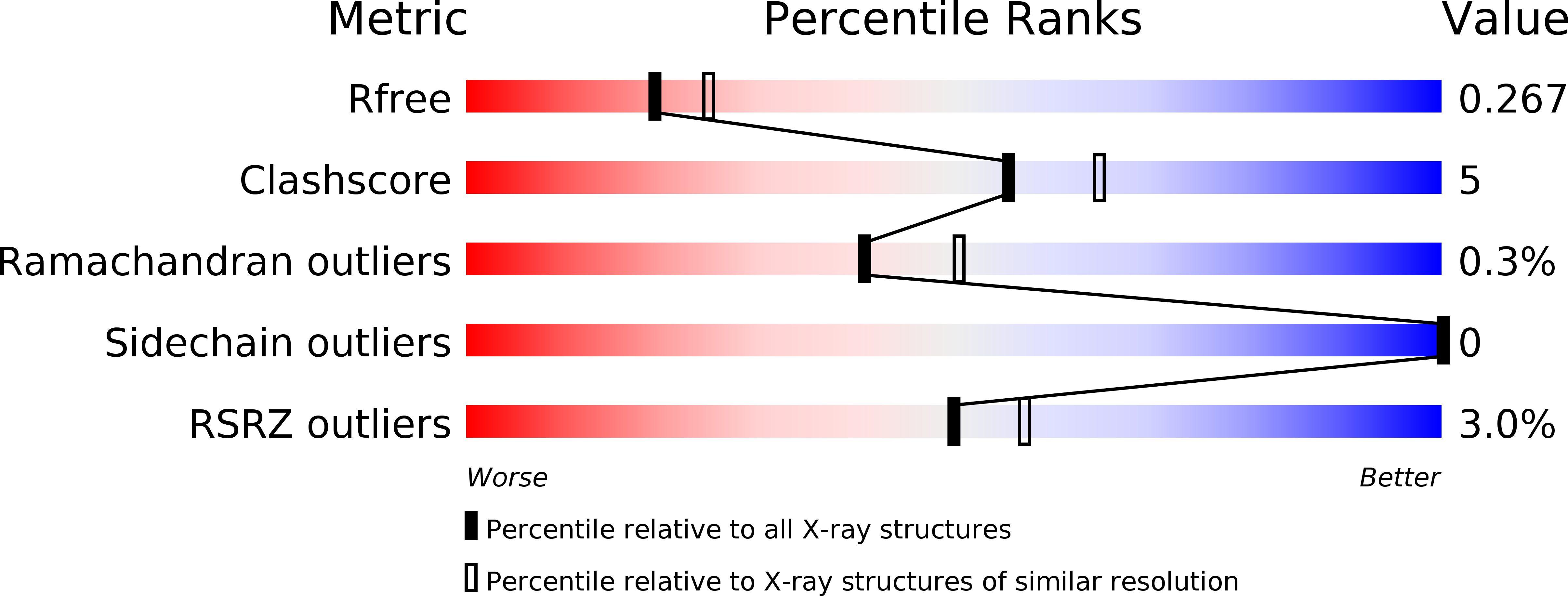
6Q8K, 6Q8P - PubMed Abstract:
Cdc2-like kinase 1 (CLK1) and dual specificity tyrosine phosphorylation-regulated kinase 1A (DYRK1A) are involved in the regulation of alternative pre-mRNA splicing. Dysregulation of this process has been linked to cancer progression and neurodegenerative diseases, making CLK1 and DYRK1A important therapeutic targets. Here we describe the synthesis of new pyrido[3,4-g]quinazoline derivatives and the evaluation of the inhibitory potencies of these compounds toward CDK5, CK1, GSK3, CLK1 and DYRK1A. Introduction of aminoalkylamino groups at the 2-position resulted in several compounds with low nanomolar affinity and selective inhibition of CLK1 and/or DYRK1A. Their evaluation on several immortalized or cancerous cell lines showed varying degree of cell viability reduction. Co-crystal structures of CLK1 with two of the most potent compounds revealed two alternative binding modes of the pyrido[3,4-g]quinazoline scaffold that can be exploited for future inhibitor design.
Organizational Affiliation:
Université Clermont Auvergne, CNRS, SIGMA Clermont, ICCF, F-63000, Clermont-Ferrand, France; Carthage University, Laboratory of Organic and Analytical Chemistry (ISEFC), Tunis, Tunisia.