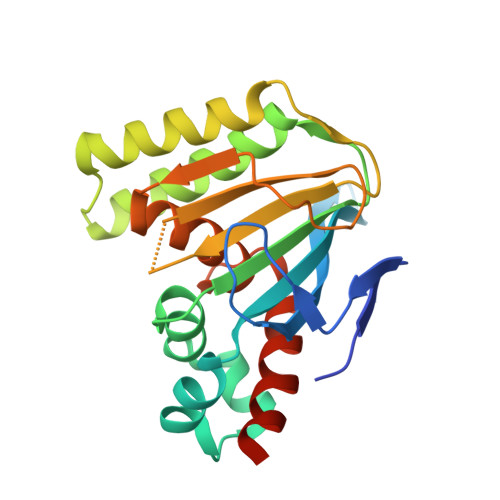
Bifunctional Substrate Activation via an Arginine Residue Drives Catalysis in Chalcone Isomerases
Burke, J.R., La Clair, J.J., Philippe, R.N., Pabis, A., Jez, J.M., Cortina, G., Kaltenbach, M., Bowman, M.E., Woods, K.B., Nelson, A.T., Tawfik, D.S., Kamerlin, S.C.L., Noel, J.P.(2019) ACS Catal