Exploitation of dihydroorotate dehydrogenase (DHODH) and p53 activation as therapeutic targets: A case study in polypharmacology.
Ladds, M.J.G.W., Popova, G., Pastor-Fernandez, A., Kannan, S., van Leeuwen, I.M.M., Hakansson, M., Walse, B., Tholander, F., Bhatia, R., Verma, C.S., Lane, D.P., Lain, S.(2020) J Biol Chem 295: 17935-17949
- PubMed: 32900849
- DOI: https://doi.org/10.1074/jbc.RA119.012056
- Primary Citation of Related Structures:
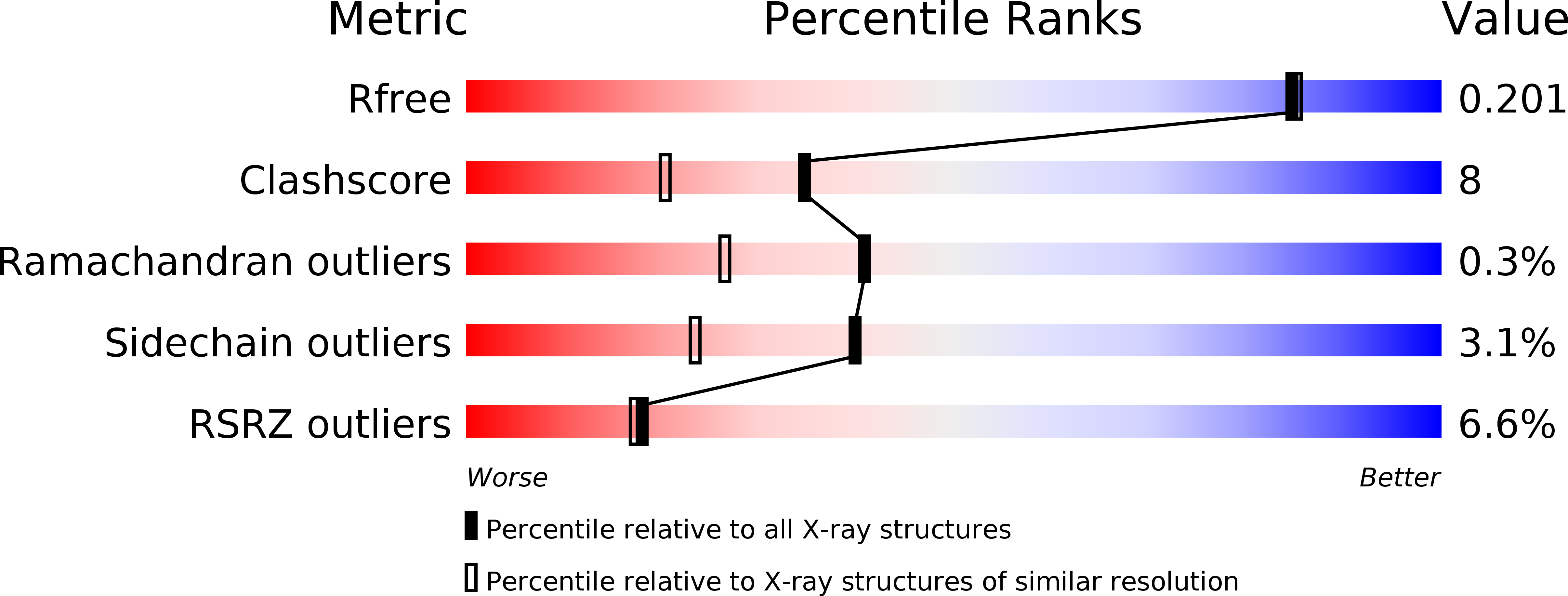
6GK0 - PubMed Abstract:
The tenovins are a frequently studied class of compounds capable of inhibiting sirtuin activity, which is thought to result in increased acetylation and protection of the tumor suppressor p53 from degradation. However, as we and other laboratories have shown previously, certain tenovins are also capable of inhibiting autophagic flux, demonstrating the ability of these compounds to engage with more than one target. In this study, we present two additional mechanisms by which tenovins are able to activate p53 and kill tumor cells in culture. These mechanisms are the inhibition of a key enzyme of the de novo pyrimidine synthesis pathway, dihydroorotate dehydrogenase (DHODH), and the blockage of uridine transport into cells. These findings hold a 3-fold significance: first, we demonstrate that tenovins, and perhaps other compounds that activate p53, may activate p53 by more than one mechanism; second, that work previously conducted with certain tenovins as SirT1 inhibitors should additionally be viewed through the lens of DHODH inhibition as this is a major contributor to the mechanism of action of the most widely used tenovins; and finally, that small changes in the structure of a small molecule can lead to a dramatic change in the target profile of the molecule even when the phenotypic readout remains static.
Organizational Affiliation:
Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet, Stockholm, Sweden; SciLifeLab, Department of Microbiology, Tumor and Cell Biology, Karolinska Institutet, Stockholm, Sweden. Electronic address: m.ladds@beatson.gla.ac.uk.