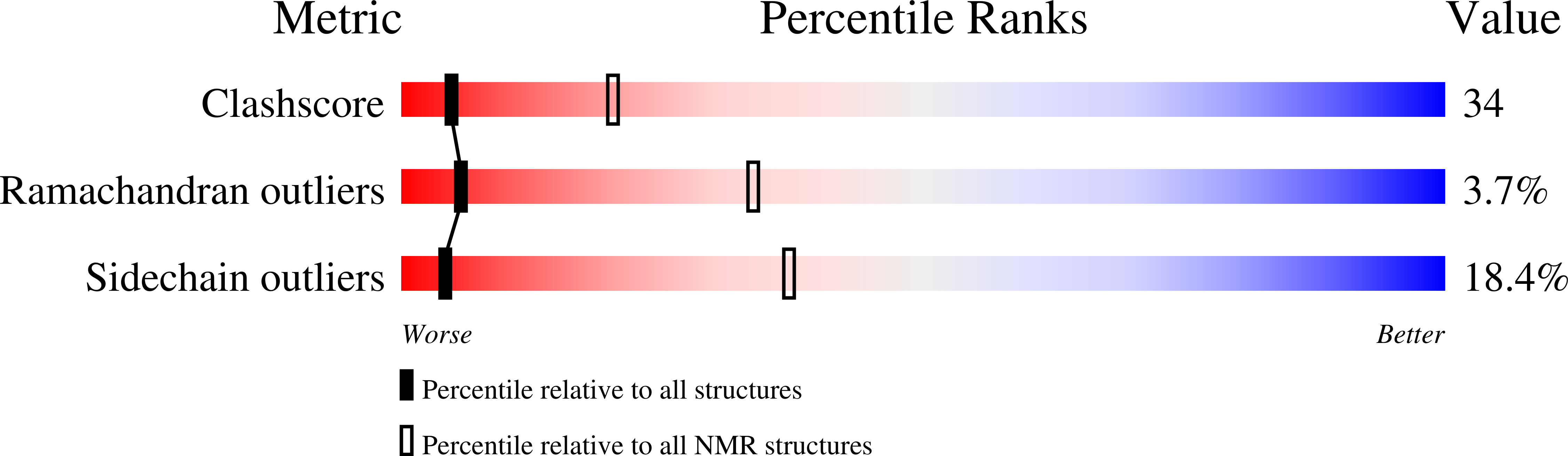1H,13C and15N resonance assignments and structure prediction of translation initiation factor 1 from Clostridium difficile.
Aguilar, F., Banaei, N., Zhang, Y.(2019) Biomol NMR Assign 13: 91-95
- PubMed: 30370502
- DOI: https://doi.org/10.1007/s12104-018-9858-8
- Primary Citation of Related Structures:
6C00 - PubMed Abstract:
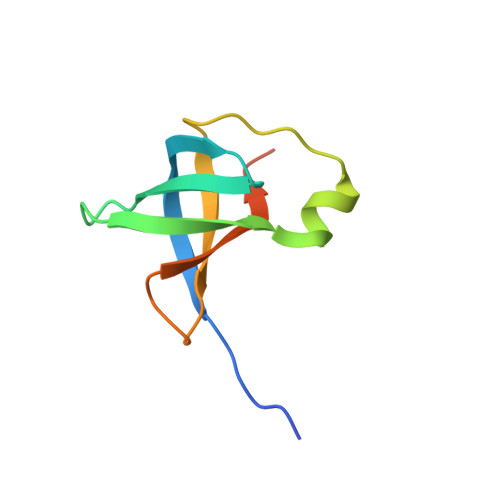
Clostridium difficile is a gram-positive, toxin-producing, anaerobic bacterium whose virulence factors and mechanisms of pathogenesis require further investigation. C. difficile infections (CDI) result in the severe and potentially fatal gastrointestinal diseases pseudomembranous colitis and toxic megacolon following extensive broad spectrum antibiotic treatment. The increasing C. difficile fatalities are a result of the bacteria's growing antibiotic resistance and consequential CDI recurrence, which led to the unmet need for new CDI treatment. Bacterial protein synthesis is an essential metabolic process and an effective target for antibacterial agents. Translation initiation factor 1 from C. difficile (Cd-IF1) is the smallest of the three initiation factors that acts to establish the 30S initiation complex to initiate translation during protein biosynthesis. Here we report the complete NMR 1 H, 13 C and 15 N chemical shift assignments of Cd-IF1 as the basis for NMR structure determination and interaction studies. Secondary structure analyses have identified five β-strands and one short α-helix arranged in the sequential order β1-β2-β3-α1-β4-β5, which is supported by 15 N-{ 1 H} heteroNOEs. The assigned chemical shifts were used to conduct structure prediction by CS-Rosetta. The predicted structure suggests that Cd-IF1 adopts the typical β-barrel structure and is composed of an oligomer-binding motif.
Organizational Affiliation:
Department of Chemistry, The University of Texas Rio Grande Valley, Edinburg, TX, USA.