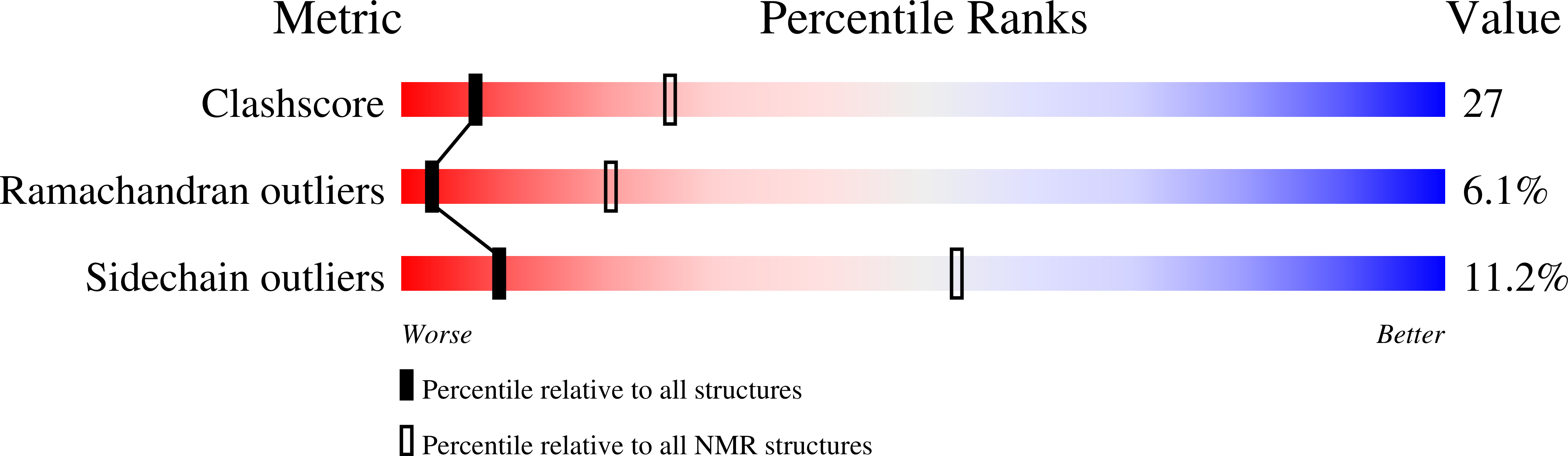Experimentally based structural model of Yih1 provides insight into its function in controlling the key translational regulator Gcn2.
Harjes, E., Jameson, G.B., Tu, Y.H., Burr, N., Loo, T.S., Goroncy, A.K., Edwards, P.J.B., Harjes, S., Munro, B., Gobl, C., Sattlegger, E., Norris, G.E.(2020) FEBS Lett
- PubMed: 33156522
- DOI: https://doi.org/10.1002/1873-3468.13990
- Primary Citation of Related Structures:
6BQI, 6U1L, 6U1O - PubMed Abstract:
Yeast impact homolog 1 (Yih1), or IMPACT in mammals, is part of a conserved regulatory module controlling the activity of General Control Nonderepressible 2 (Gcn2), a protein kinase that regulates protein synthesis. Yih1/IMPACT is implicated not only in many essential cellular processes, such as neuronal development, immune system regulation and the cell cycle, but also in cancer. Gcn2 must bind to Gcn1 in order to impair the initiation of protein translation. Yih1 hinders this key Gcn1-Gcn2 interaction by binding to Gcn1, thus preventing Gcn2-mediated inhibition of protein synthesis. Here, we solved the structures of the two domains of Saccharomyces cerevisiae Yih1 separately using Nuclear Magnetic Resonance and determined the relative positions of the two domains using a range of biophysical methods. Our findings support a compact structural model of Yih1 in which the residues required for Gcn1 binding are buried in the interface. This model strongly implies that Yih1 undergoes a large conformational rearrangement from a latent closed state to a primed open state to bind Gcn1. Our study provides structural insight into the interactions of Yih1 with partner molecules.
Organizational Affiliation:
School of Fundamental Sciences, Massey University, Palmerston North, New Zealand.