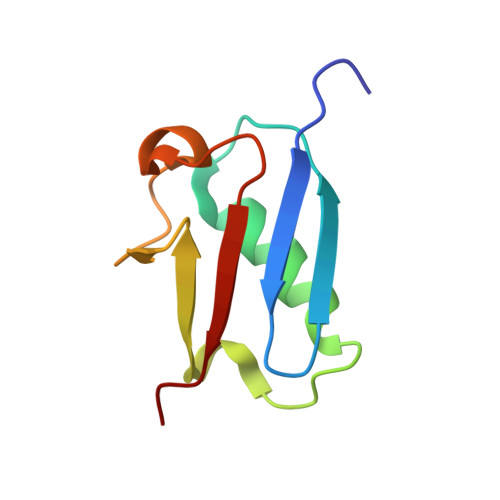
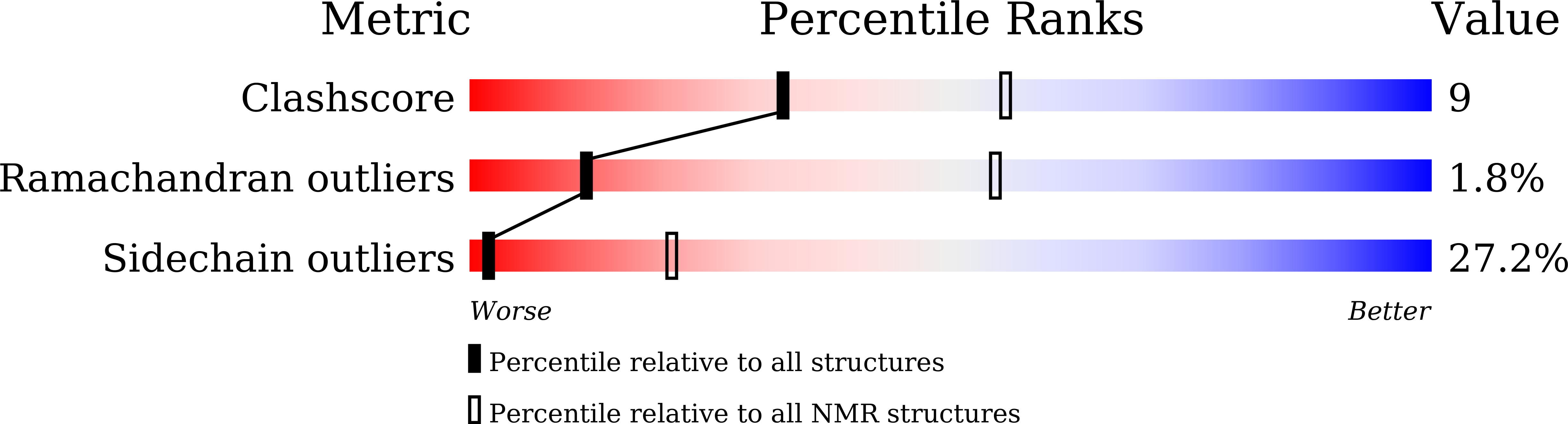Solution structure of a ubiquitin-like protein from Trypanosoma brucei
Mi, J., Zhang, J., Liao, S., Tu, X.(2018) Protein Sci 27: 1831-1836
- PubMed: 30058168
- DOI: https://doi.org/10.1002/pro.3492
- Primary Citation of Related Structures:
5ZMB - PubMed Abstract:
Ubiquitin-like proteins, similar to ubiquitin, can either exist freely or be covalently attached to other proteins via an enzymatic cascade. The ubiquitin-like proteins play roles in multiple biological processes including transcription, stress responses, DNA repair and so on. In this study, a novel ubiquitin-like protein (TbUbl11) was identified in Trypanosoma brucei. The solution structure of TbUbl11 was solved by NMR spectroscopy. TbUbl11 adopts a conserved β-grasp fold composed by a five-stranded β-sheet curling around a central α-helix, similar to other ubiquitin-like proteins. Meanwhile, some differences between TbUbl11 and other ubiquitin-like proteins were also identified. Additionally, we revealed that TbUbl11 is located in the whole cell body of procyclic-form T. brucei.
Organizational Affiliation:
Hefei National Laboratory for Physical Science at Microscale and School of Life Science, University of Science and Technology of China, Hefei, Anhui, 230026, People's Republic of China.