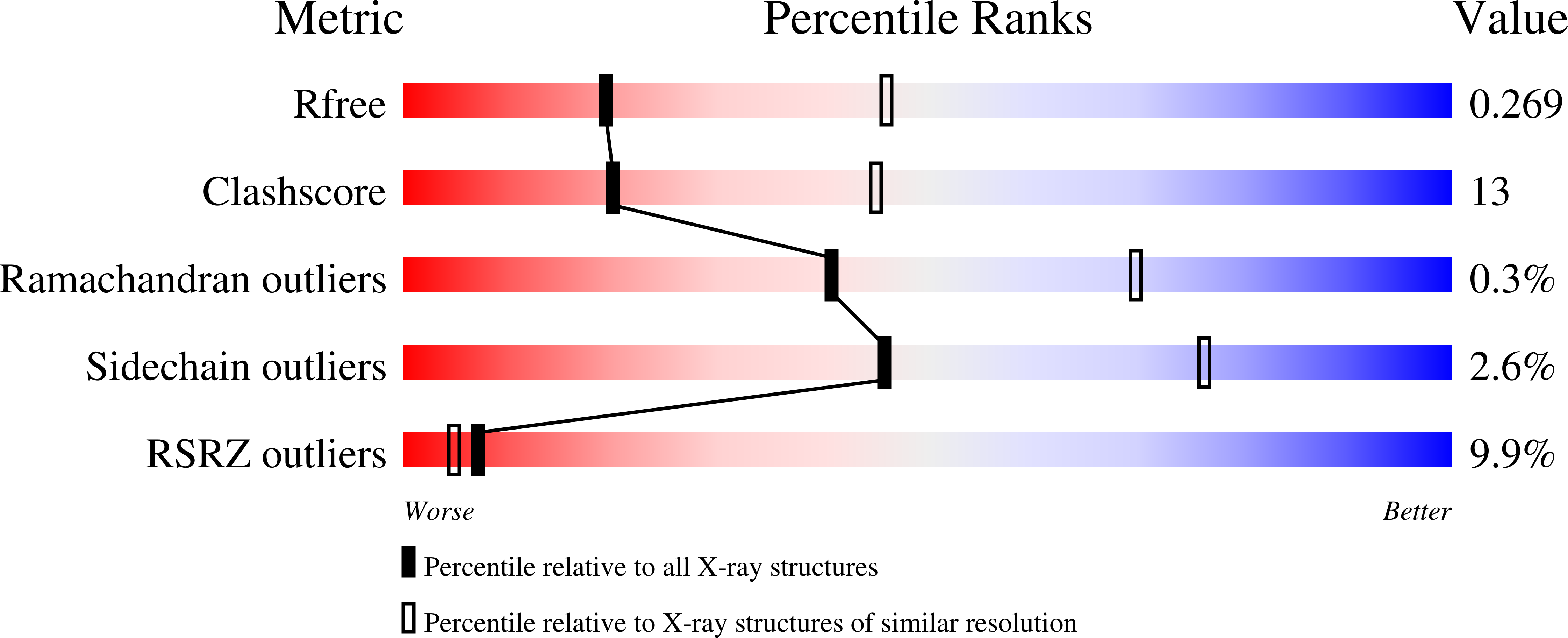
Mechanism of Substrate Translocation in an Alternating Access Transporter.
Latorraca, N.R., Fastman, N.M., Venkatakrishnan, A.J., Frommer, W.B., Dror, R.O., Feng, L.(2017) Cell 169: 96-107.e12
- PubMed: 28340354
- DOI: https://doi.org/10.1016/j.cell.2017.03.010
- Primary Citation of Related Structures:
5UHQ, 5UHS - PubMed Abstract:
Transporters shuttle molecules across cell membranes by alternating among distinct conformational states. Fundamental questions remain about how transporters transition between states and how such structural rearrangements regulate substrate translocation. Here, we capture the translocation process by crystallography and unguided molecular dynamics simulations, providing an atomic-level description of alternating access transport. Simulations of a SWEET-family transporter initiated from an outward-open, glucose-bound structure reported here spontaneously adopt occluded and inward-open conformations. Strikingly, these conformations match crystal structures, including our inward-open structure. Mutagenesis experiments further validate simulation predictions. Our results reveal that state transitions are driven by favorable interactions formed upon closure of extracellular and intracellular "gates" and by an unfavorable transmembrane helix configuration when both gates are closed. This mechanism leads to tight allosteric coupling between gates, preventing them from opening simultaneously. Interestingly, the substrate appears to take a "free ride" across the membrane without causing major structural rearrangements in the transporter.
Organizational Affiliation:
Biophysics Program, Stanford University, Stanford, CA 94305, USA; Department of Computer Science, Stanford University, Stanford, CA 94305, USA; Department of Molecular and Cellular Physiology, Stanford University School of Medicine, Stanford, CA 94305, USA; Institute for Computational and Mathematical Engineering, Stanford University, Stanford, CA 94305, USA.