Binding Properties of Split tRNA to the C-terminal Domain of Methionyl-tRNA Synthetase of Nanoarchaeum equitans.
Suzuki, H., Kaneko, A., Yamamoto, T., Nambo, M., Hirasawa, I., Umehara, T., Yoshida, H., Park, S.Y., Tamura, K.(2017) J Mol Evol 84: 267-278
- PubMed: 28589220
- DOI: https://doi.org/10.1007/s00239-017-9796-6
- Primary Citation of Related Structures:
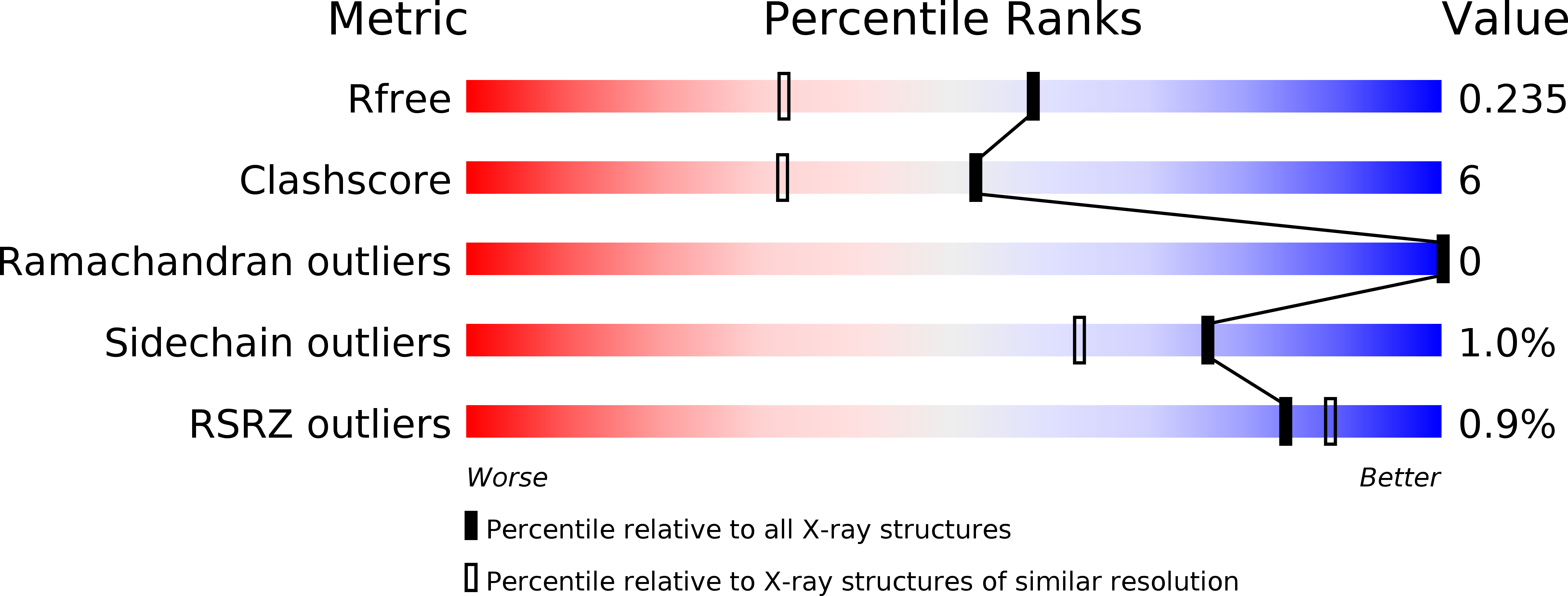
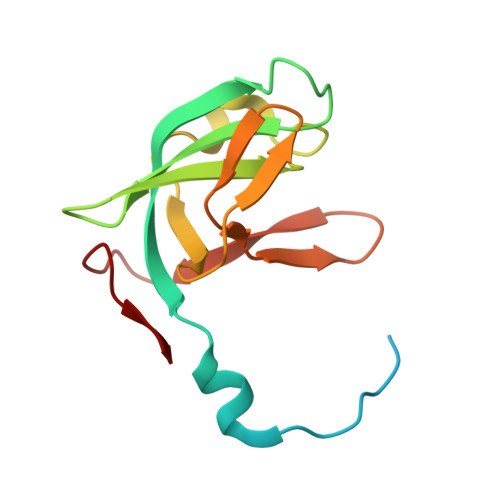
5H34 - PubMed Abstract:
The C-terminal domain of methionyl-tRNA synthetase (MetRS-C) from Nanoarchaeum equitans is homologous to a tRNA-binding protein consisting of 111 amino acids (Trbp111) from Aquifex aeolicus. The crystal structure of MetRS-C showed that it existed as a homodimer, and that each monomer possessed an oligonucleotide/oligosaccharide-binding fold (OB-fold). Analysis using a quartz crystal microbalance indicated that MetRS-C freshly isolated from N. equitans was bound to tRNA. However, binding of the split 3'-half tRNA species was stronger than that of the 5'-half species. The T-loop and the 3'-end regions of the split 3'-half tRNA were found to be responsible for the binding. The minimum structure for binding to MetRS-C might be a minihelix-like stem-loop with single-stranded 3'-terminus. After successive duplications of such a small hairpin structure with the assistance of a Trbp-like structure, the interaction of the T-loop region of the 3'-half with a Trbp-like structure could have been evolutionarily replaced by RNA-RNA interactions, along with many combinational tertiary interactions, to form the modern tRNA structure.
Organizational Affiliation:
Department of Biological Science and Technology, Tokyo University of Science, 6-3-1 Niijuku, Katsushika-ku, Tokyo, 125-8585, Japan.