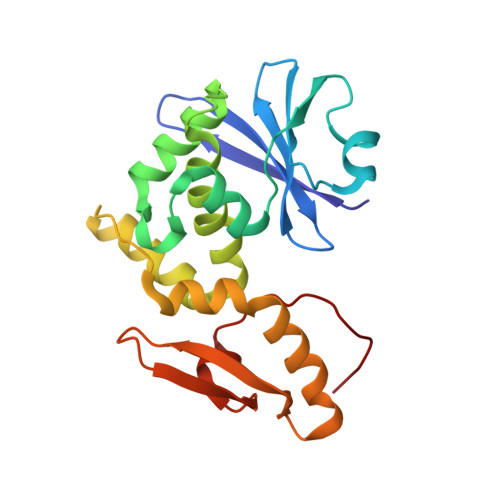
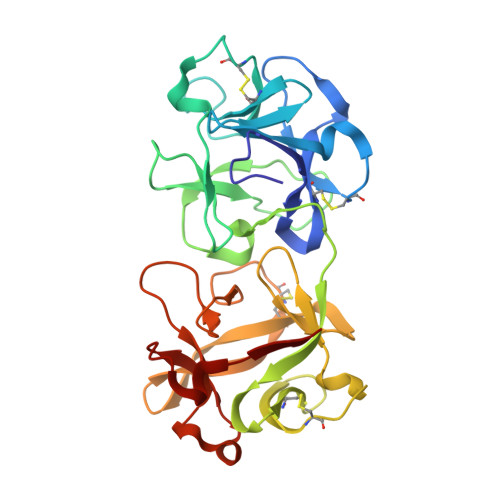
The sequence and structure of snake gourd (Trichosanthes anguina) seed lectin, a three-chain nontoxic homologue of type II RIPs.
Sharma, A., Pohlentz, G., Bobbili, K.B., Jeyaprakash, A.A., Chandran, T., Mormann, M., Swamy, M.J., Vijayan, M.(2013) Acta Crystallogr D Biol Crystallogr 69: 1493-1503
- PubMed: 23897472
- DOI: https://doi.org/10.1107/S0907444913010020
- Primary Citation of Related Structures:
4HR6 - PubMed Abstract:
The sequence and structure of snake gourd seed lectin (SGSL), a nontoxic homologue of type II ribosome-inactivating proteins (RIPs), have been determined by mass spectrometry and X-ray crystallography, respectively. As in type II RIPs, the molecule consists of a lectin chain made up of two β-trefoil domains. The catalytic chain, which is connected through a disulfide bridge to the lectin chain in type II RIPs, is cleaved into two in SGSL. However, the integrity of the three-dimensional structure of the catalytic component of the molecule is preserved. This is the first time that a three-chain RIP or RIP homologue has been observed. A thorough examination of the sequence and structure of the protein and of its interactions with the bound methyl-α-galactose indicate that the nontoxicity of SGSL results from a combination of changes in the catalytic and the carbohydrate-binding sites. Detailed analyses of the sequences of type II RIPs of known structure and their homologues with unknown structure provide valuable insights into the evolution of this class of proteins. They also indicate some variability in carbohydrate-binding sites, which appears to contribute to the different levels of toxicity exhibited by lectins from various sources.
Organizational Affiliation:
Molecular Biophysics Unit, Indian Institute of Science, Bangalore 560 012, Karnataka, India.