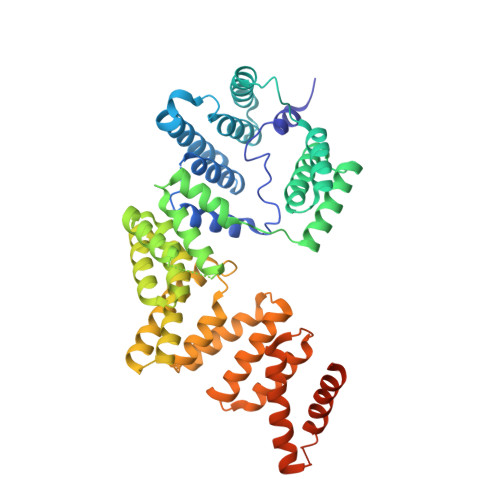
Crystal structure of ISG54 reveals a novel RNA binding structure and potential functional mechanisms.
Yang, Z., Liang, H., Zhou, Q., Li, Y., Chen, H., Ye, W., Chen, D., Fleming, J., Shu, H., Liu, Y.(2012) Cell Res 22: 1328-1338
- PubMed: 22825553
- DOI: https://doi.org/10.1038/cr.2012.111
- Primary Citation of Related Structures:
4G1T - PubMed Abstract:
Interferon-stimulated gene 56 (ISG56) family members play important roles in blocking viral replication and regulating cellular functions, however, their underlying molecular mechanisms are largely unclear. Here, we present the crystal structure of ISG54, an ISG56 family protein with a novel RNA-binding structure. The structure shows that ISG54 monomers have 9 tetratricopeptide repeat-like motifs and associate to form domain-swapped dimers. The C-terminal part folds into a super-helical structure and has an extensively positively-charged nucleotide-binding channel on its inner surface. EMSA results show that ISG54 binds specifically to some RNAs, such as adenylate uridylate (AU)-rich RNAs, with or without 5' triphosphorylation. Mutagenesis and functional studies show that this RNA-binding ability is important to its antiviral activity. Our results suggest a new mechanism underlying the antiviral activity of this interferon-inducible gene 56 family member.
Organizational Affiliation:
State Key Laboratory of Biomacromolecules, Institute of Biophysics, Chinese Academy of Sciences, 15 Datun Road, Chaoyang District, Beijing 100101, China.