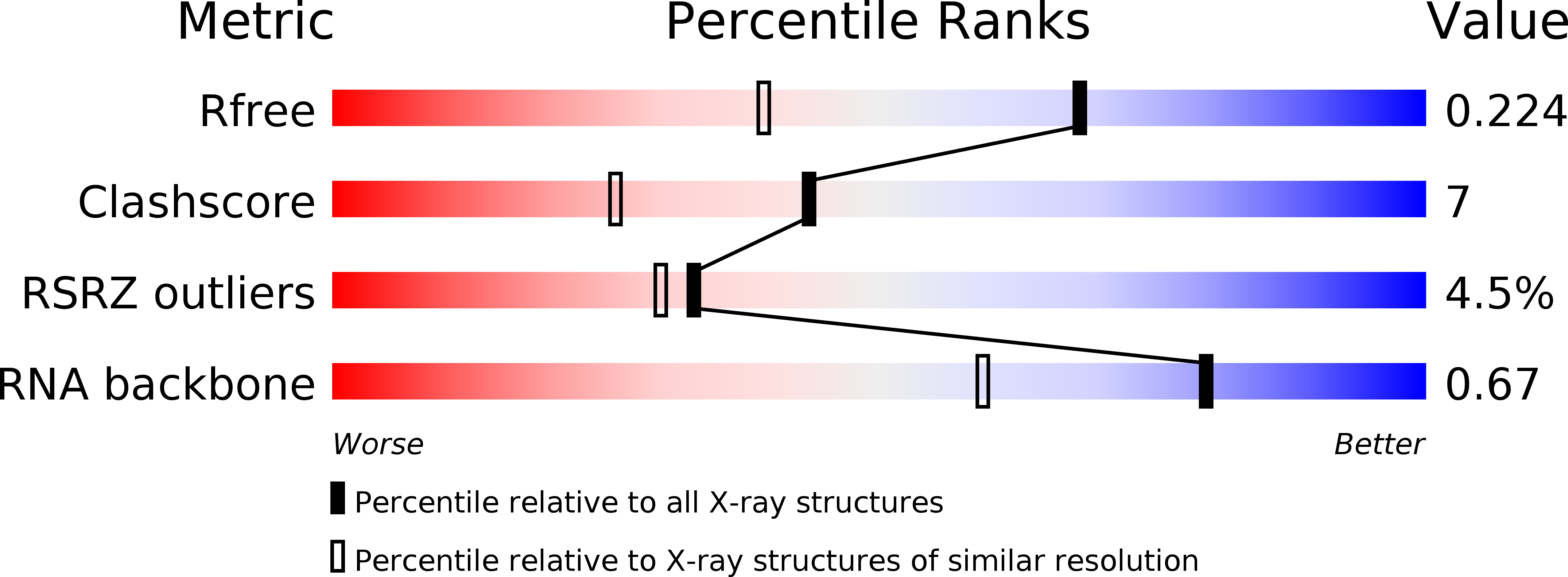
Nucleotides Adjacent to the Ligand-Binding Pocket are Linked to Activity Tuning in the Purine Riboswitch.
Stoddard, C.D., Widmann, J., Trausch, J.J., Marcano-Velazquez, J.G., Knight, R., Batey, R.T.(2013) J Mol Biol 425: 1596-1611
- PubMed: 23485418
- DOI: https://doi.org/10.1016/j.jmb.2013.02.023
- Primary Citation of Related Structures:
4FEJ, 4FEL, 4FEN, 4FEO, 4FEP - PubMed Abstract:
Direct sensing of intracellular metabolite concentrations by riboswitch RNAs provides an economical and rapid means to maintain metabolic homeostasis. Since many organisms employ the same class of riboswitch to control different genes or transcription units, it is likely that functional variation exists in riboswitches such that activity is tuned to meet cellular needs. Using a bioinformatic approach, we have identified a region of the purine riboswitch aptamer domain that displays conservation patterns linked to riboswitch activity. Aptamer domain compositions within this region can be divided into nine classes that display a spectrum of activities. Naturally occurring compositions in this region favor rapid association rate constants and slow dissociation rate constants for ligand binding. Using X-ray crystallography and chemical probing, we demonstrate that both the free and bound states are influenced by the composition of this region and that modest sequence alterations have a dramatic impact on activity. The introduction of non-natural compositions result in the inability to regulate gene expression in vivo, suggesting that aptamer domain activity is highly plastic and thus readily tunable to meet cellular needs.
Organizational Affiliation:
Department of Chemistry and Biochemistry, 596 UCB, University of Colorado, Boulder, CO 80309-0596, USA.