The molecular architecture of the yeast spindle pole body core determined by Bayesian integrative modeling.
Viswanath, S., Bonomi, M., Kim, S.J., Klenchin, V.A., Taylor, K.C., Yabut, K.C., Umbreit, N.T., Van Epps, H.A., Meehl, J., Jones, M.H., Russel, D., Velazquez-Muriel, J.A., Winey, M., Rayment, I., Davis, T.N., Sali, A., Muller, E.G.(2017) Mol Biol Cell 28: 3298-3314
- PubMed: 28814505
- DOI: https://doi.org/10.1091/mbc.E17-06-0397
- Primary Citation of Related Structures:
4DS7 - PubMed Abstract:
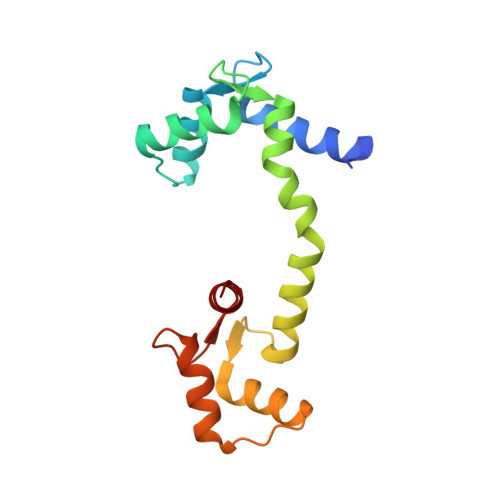
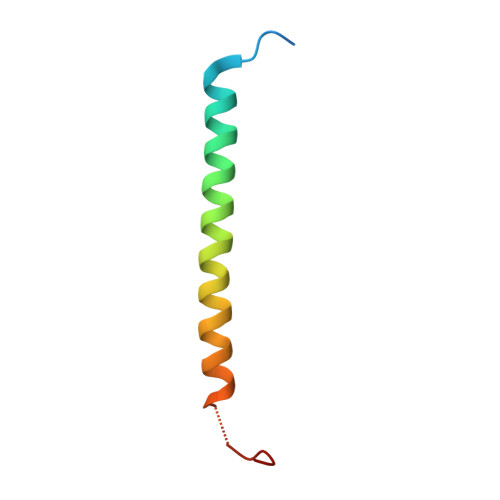
Microtubule-organizing centers (MTOCs) form, anchor, and stabilize the polarized network of microtubules in a cell. The central MTOC is the centrosome that duplicates during the cell cycle and assembles a bipolar spindle during mitosis to capture and segregate sister chromatids. Yet, despite their importance in cell biology, the physical structure of MTOCs is poorly understood. Here we determine the molecular architecture of the core of the yeast spindle pole body (SPB) by Bayesian integrative structure modeling based on in vivo fluorescence resonance energy transfer (FRET), small-angle x-ray scattering (SAXS), x-ray crystallography, electron microscopy, and two-hybrid analysis. The model is validated by several methods that include a genetic analysis of the conserved PACT domain that recruits Spc110, a protein related to pericentrin, to the SPB. The model suggests that calmodulin can act as a protein cross-linker and Spc29 is an extended, flexible protein. The model led to the identification of a single, essential heptad in the coiled-coil of Spc110 and a minimal PACT domain. It also led to a proposed pathway for the integration of Spc110 into the SPB.
Organizational Affiliation:
Department of Bioengineering and Therapeutic Sciences, Department of Pharmaceutical Chemistry, California Institute for Quantitative Biosciences, University of California, San Francisco, San Francisco, CA 94158.