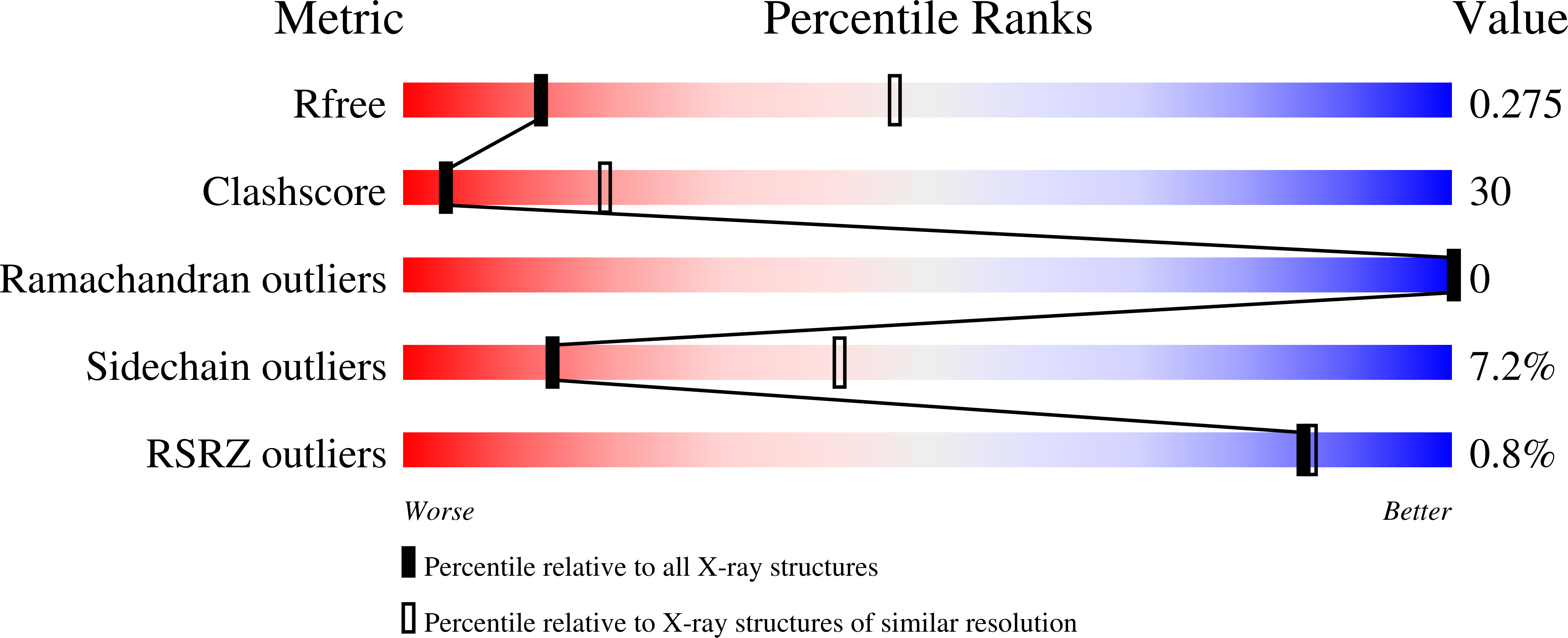
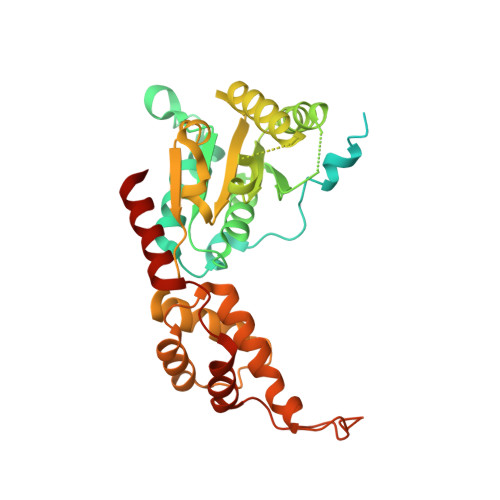
Crystal structure of the human spastin AAA domain.
Taylor, J.L., White, S.R., Lauring, B., Kull, F.J.(2012) J Struct Biol 179: 133-137
- PubMed: 22446388
- DOI: https://doi.org/10.1016/j.jsb.2012.03.002
- Primary Citation of Related Structures:
3VFD - PubMed Abstract:
Hereditary spastic paraplegia (HSP) is a motor neuron disease caused by a progressive degeneration of the motor axons of the corticospinal tract. Point mutations or exon deletions in the microtubule-severing ATPase, spastin, are responsible for approximately 40% of cases of autosomal dominant HSP. Here, we report the 3.3 Å X-ray crystal structure of a hydrolysis-deficient mutant (E442Q) of the human spastin protein AAA domain. This structure is analyzed in the context of the existing Drosophila melanogaster spastin AAA domain structure and crystal structures of other closely related proteins in order to build a more unifying framework for understanding the structural features of this group of microtubule-severing ATPases.
Organizational Affiliation:
Department of Chemistry, Dartmouth College, Hanover, NH 03755, USA.