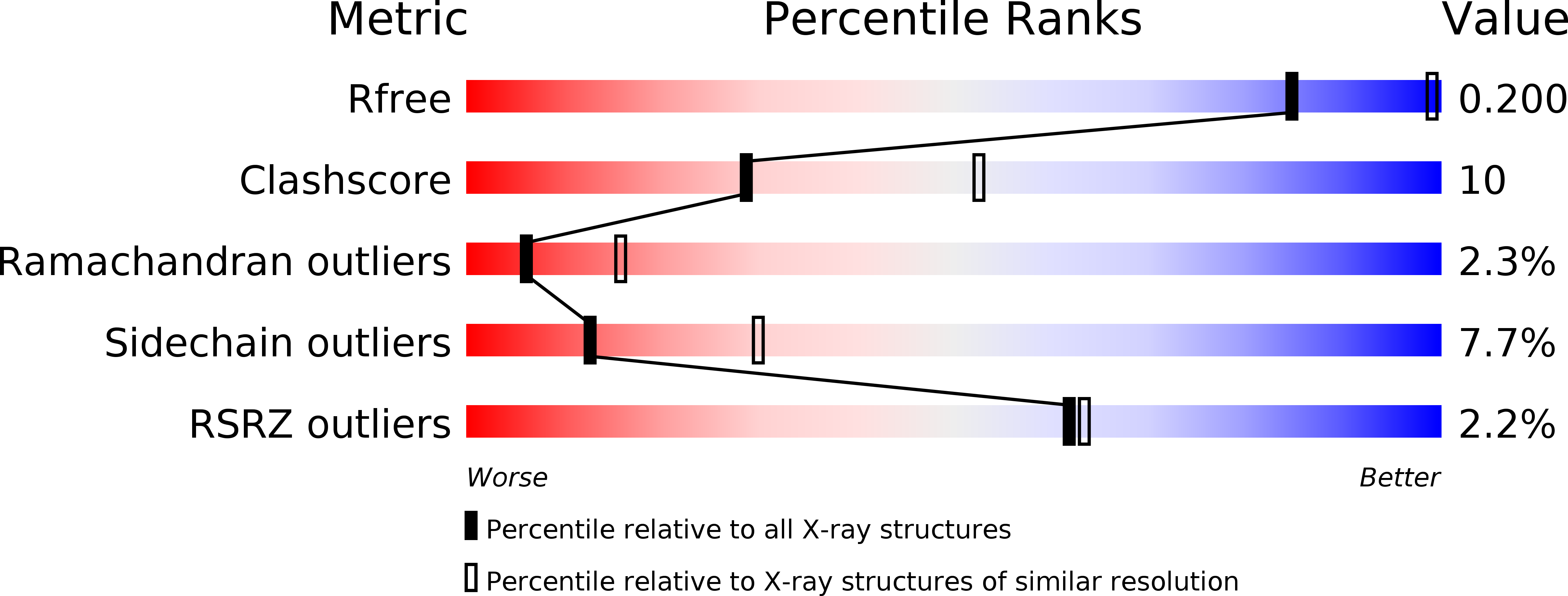
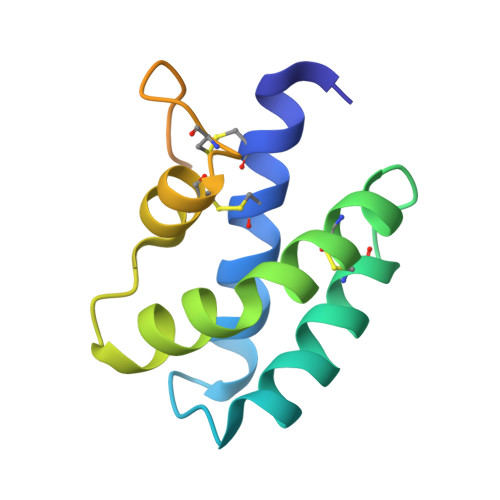
Insights into the membrane interactions of the saposin-like proteins Na-SLP-1 and Ac-SLP-1 from human and dog hookworm.
Willis, C., Wang, C.K., Osman, A., Simon, A., Pickering, D., Mulvenna, J., Riboldi-Tunicliffe, A., Jones, M.K., Loukas, A., Hofmann, A.(2011) PLoS One 6: e25369-e25369
- PubMed: 21991310
- DOI: https://doi.org/10.1371/journal.pone.0025369
- Primary Citation of Related Structures:
3S63, 3S64 - PubMed Abstract:
Saposin-like proteins (SAPLIPs) from soil-transmitted helminths play pivotal roles in host-pathogen interactions and have a high potential as targets for vaccination against parasitic diseases. We have identified two non-orthologous SAPLIPs from human and dog hookworm, Na-SLP-1 and Ac-SLP-1, and solved their three-dimensional crystal structures. Both proteins share the property of membrane binding as monitored by liposome co-pelleting assays and monolayer adsorption. Neither SAPLIP displayed any significant haemolytic or bactericidal activity. Based on the structural information, as well as the results from monolayer adsorption, we propose models of membrane interactions for both SAPLIPs. Initial membrane contact of the monomeric Na-SLP-1 is most likely by electrostatic interactions between the membrane surface and a prominent basic surface patch. In case of the dimeric Ac-SLP-1, membrane interactions are most likely initiated by a unique tryptophan residue that has previously been implicated in membrane interactions in other SAPLIPs.
Organizational Affiliation:
Parasite Cell Biology, Queensland Institute of Medical Research, Herston, Queensland, Australia.