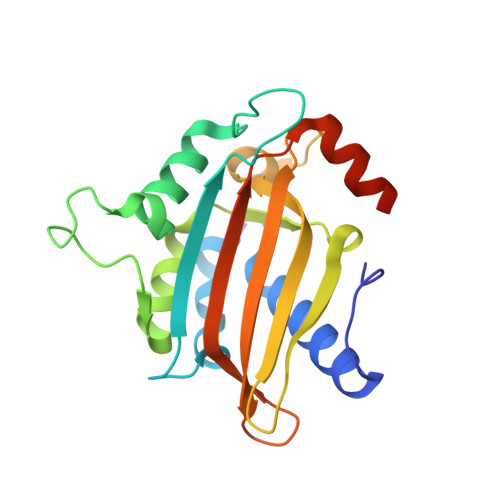
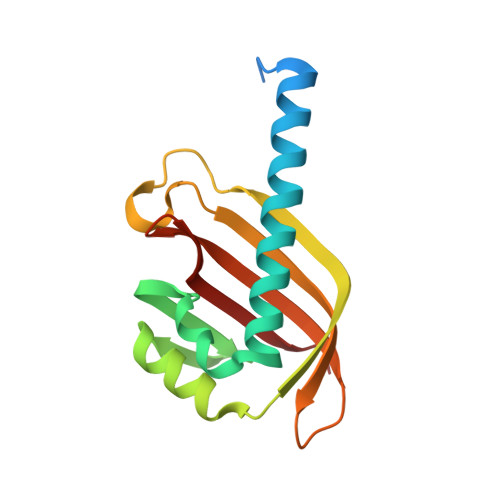
The structure of the NXF2/NXT1 heterodimeric complex reveals the combined specificity and versatility of the NTF2-like fold.
Kerkow, D.E., Carmel, A.B., Menichelli, E., Ambrus, G., Hills, R.D., Gerace, L., Williamson, J.R.(2012) J Mol Biol 415: 649-665
- PubMed: 22123199
- DOI: https://doi.org/10.1016/j.jmb.2011.11.027
- Primary Citation of Related Structures:
3NV0 - PubMed Abstract:
NXF1-like members of the NXF (nuclear export factor) family orchestrate bulk nuclear export of mRNA, while functionally distinct NXF variant proteins carry out separate substrate-specific and tissue-specific RNA regulation. Metazoan organisms possess at least one NXF1-like gene and one or more NXF variant genes. Heterodimerization of both proteins with the NXT (NTF2-related export) protein is central to NXF family function; however, given the multiplicity of NXF/NXT complexes, the specificity and mechanism of heterodimerization remain unclear. Here, we report the structural and functional analyses of the Caenorhabditis elegans NXF variant ceNXF2 bound to ceNXT1. Contacts crucial for NXF/NXT heterodimer stability and specificity, including a probable site for phosphoregulation, have been identified. The ceNXF2 NTF2 domain bears at least two nucleoporin (Nup) binding pockets necessary for the colocalization of ceNXF2/ceNXT1 at the nuclear envelope. Unexpectedly, one Nup binding pocket is formed at the heterodimer interface of the ceNXF2/ceNXT1 complex, demonstrating that NXT binding directly regulates NXF function.
Organizational Affiliation:
Department of Molecular Biology, Department of Chemistry, The Skaggs Institute for Chemical Biology, The Scripps Research Institute, La Jolla, CA 92037, USA.