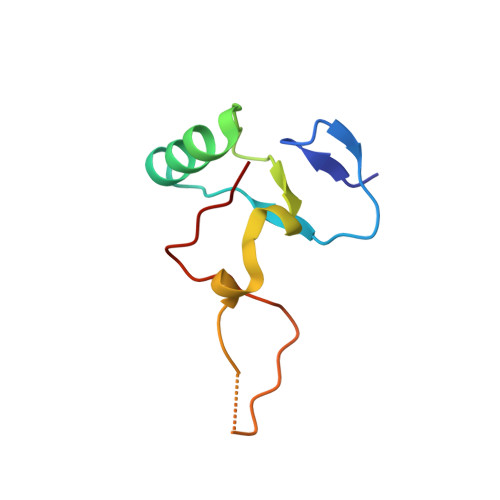
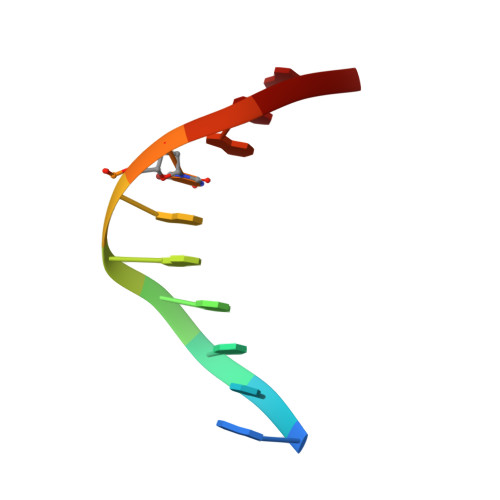
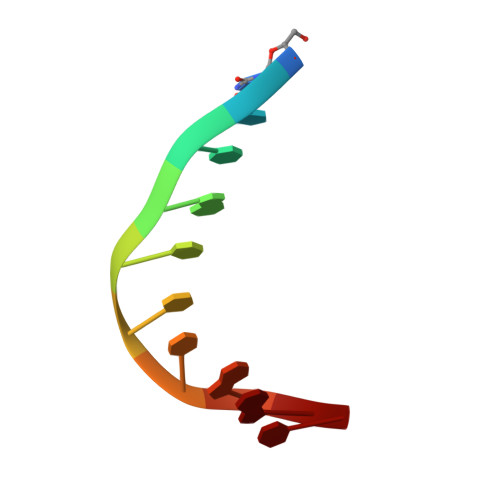
THAP proteins target specific DNA sites through bipartite recognition of adjacent major and minor grooves.
Sabogal, A., Lyubimov, A.Y., Corn, J.E., Berger, J.M., Rio, D.C.(2010) Nat Struct Mol Biol 17: 117-123
- PubMed: 20010837
- DOI: https://doi.org/10.1038/nsmb.1742
- Primary Citation of Related Structures:
3KDE - PubMed Abstract:
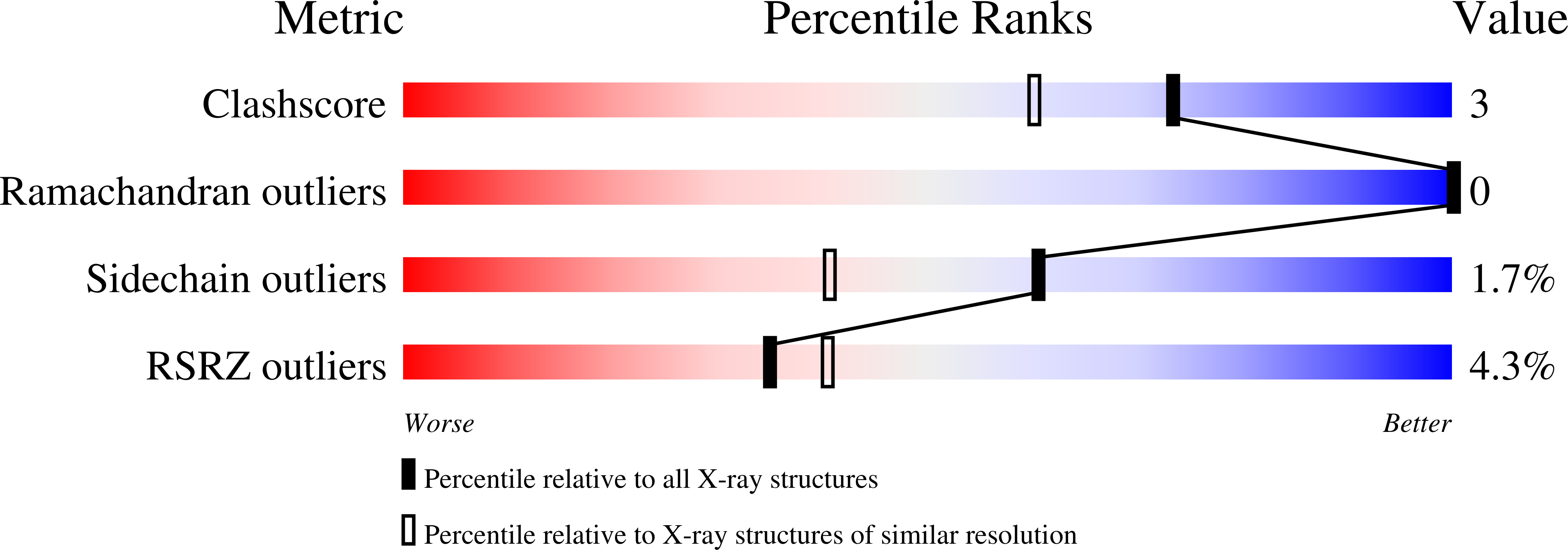
THAP-family C(2)CH zinc-coordinating DNA-binding proteins function in diverse eukaryotic cellular processes, such as transposition, transcriptional repression, stem-cell pluripotency, angiogenesis and neurological function. To determine the molecular basis for sequence-specific DNA recognition by THAP proteins, we solved the crystal structure of the Drosophila melanogaster P element transposase THAP domain (DmTHAP) in complex with a natural 10-base-pair site. In contrast to C(2)H(2) zinc fingers, DmTHAP docks a conserved beta-sheet into the major groove and a basic C-terminal loop into the adjacent minor groove. We confirmed specific protein-DNA interactions by mutagenesis and DNA-binding assays. Sequence analysis of natural and in vitro-selected binding sites suggests that several THAPs (DmTHAP and human THAP1 and THAP9) recognize a bipartite TXXGGGX(A/T) consensus motif; homology suggests THAP proteins bind DNA through a bipartite interaction. These findings reveal the conserved mechanisms by which THAP-family proteins engage specific chromosomal target elements.
Organizational Affiliation:
Department of Molecular and Cell Biology, University of California, Berkeley, Berkeley, California, USA.