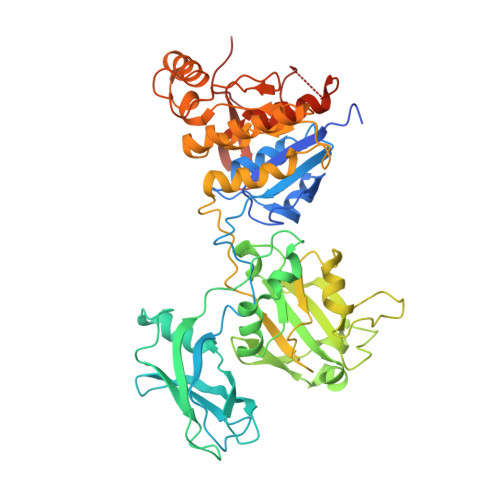
The tail sheath structure of bacteriophage T4: a molecular machine for infecting bacteria.
Aksyuk, A.A., Leiman, P.G., Kurochkina, L.P., Shneider, M.M., Kostyuchenko, V.A., Mesyanzhinov, V.V., Rossmann, M.G.(2009) EMBO J 28: 821-829
- PubMed: 19229296
- DOI: https://doi.org/10.1038/emboj.2009.36
- Primary Citation of Related Structures:
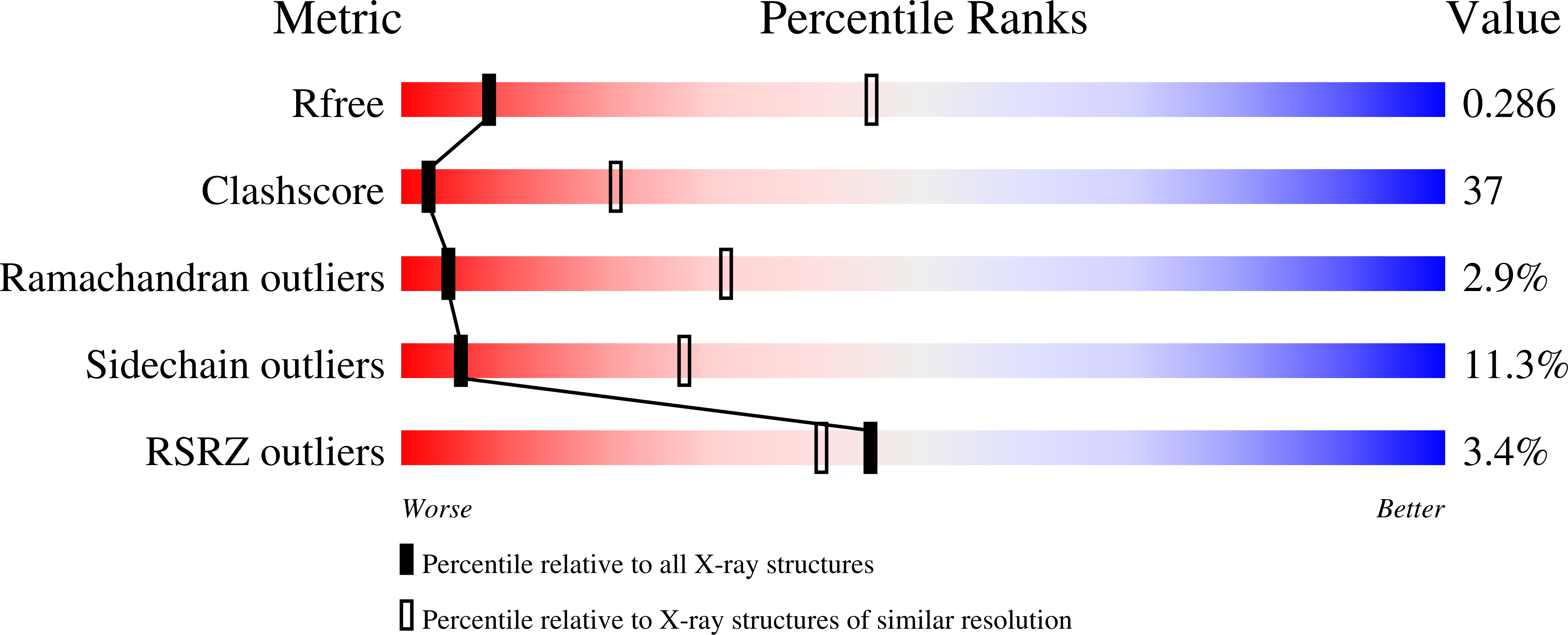
3FO8, 3FOA, 3FOH, 3FOI - PubMed Abstract:
The contractile tail of bacteriophage T4 is a molecular machine that facilitates very high viral infection efficiency. Its major component is a tail sheath, which contracts during infection to less than half of its initial length. The sheath consists of 138 copies of the tail sheath protein, gene product (gp) 18, which surrounds the central non-contractile tail tube. The contraction of the sheath drives the tail tube through the outer membrane, creating a channel for the viral genome delivery. A crystal structure of about three quarters of gp18 has been determined and was fitted into cryo-electron microscopy reconstructions of the tail sheath before and after contraction. It was shown that during contraction, gp18 subunits slide over each other with no apparent change in their structure.
Organizational Affiliation:
Department of Biological Sciences, Purdue University, West Lafayette, IN 47907-2054, USA.