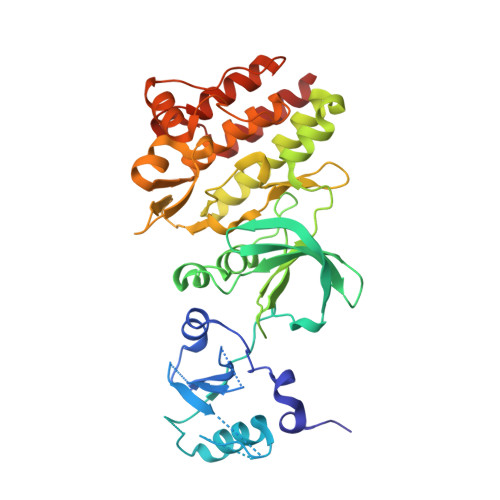
Structural Coupling of SH2-Kinase Domains Links Fes and Abl Substrate Recognition and Kinase Activation
Filippakopoulos, P., Kofler, M., Hantschel, O., Gish, G.D., Grebien, F., Salah, E., Neudecker, P., Kay, L.E., Turk, B.E., Superti-Furga, G., Pawson, T., Knapp, S.(2008) Cell 134: 793-803
- PubMed: 18775312
- DOI: https://doi.org/10.1016/j.cell.2008.07.047
- Primary Citation of Related Structures:
3BKB, 3CBL, 3CD3 - PubMed Abstract:
The SH2 domain of cytoplasmic tyrosine kinases can enhance catalytic activity and substrate recognition, but the molecular mechanisms by which this is achieved are poorly understood. We have solved the structure of the prototypic SH2-kinase unit of the human Fes tyrosine kinase, which appears specialized for positive signaling. In its active conformation, the SH2 domain tightly interacts with the kinase N-terminal lobe and positions the kinase alphaC helix in an active configuration through essential packing and electrostatic interactions. This interaction is stabilized by ligand binding to the SH2 domain. Our data indicate that Fes kinase activation is closely coupled to substrate recognition through cooperative SH2-kinase-substrate interactions. Similarly, we find that the SH2 domain of the active Abl kinase stimulates catalytic activity and substrate phosphorylation through a distinct SH2-kinase interface. Thus, the SH2 and catalytic domains of active Fes and Abl pro-oncogenic kinases form integrated structures essential for effective tyrosine kinase signaling.
Organizational Affiliation:
Structural Genomics Consortium, University of Oxford, Old Road Campus, Roosevelt Drive, Oxford OX3 7DQ, UK.