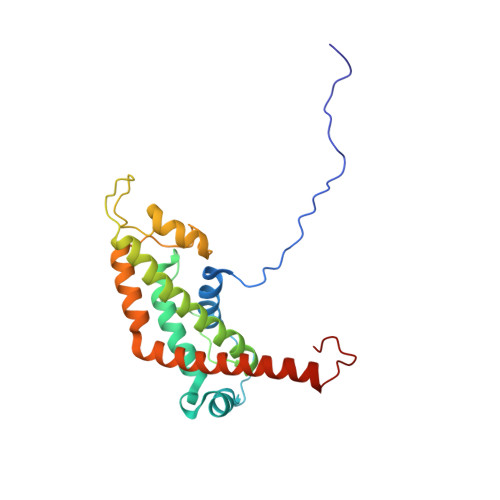
Structural and Functional Characterization of Bc28.1, Major Erythrocyte-binding Protein from Babesia canis Merozoite Surface.
Yang, Y.S., Murciano, B., Moubri, K., Cibrelus, P., Schetters, T., Gorenflot, A., Delbecq, S., Roumestand, C.(2012) J Biol Chem 287: 9495-9508
- PubMed: 22294693
- DOI: https://doi.org/10.1074/jbc.M111.260745
- Primary Citation of Related Structures:
2LCU - PubMed Abstract:
Babesiosis (formerly known as piroplasmosis) is a tick-borne disease caused by the intraerythrocytic development of protozoa parasites from the genus Babesia. Like Plasmodium falciparum, the agent of malaria, or Toxoplasma gondii, responsible for human toxoplasmosis, Babesia belongs to the Apicomplexa family. Babesia canis is the agent of the canine babesiosis in Europe. Clinical manifestations of this disease range from mild to severe and possibly lead to death by multiple organ failure. The identification and characterization of parasite surface proteins represent major goals, both for the understanding of the Apicomplexa invasion process and for the vaccine potential of such antigens. Indeed, we have already shown that Bd37, the major antigenic adhesion protein from Babesia divergens, the agent of bovine babesiosis, was able to induce complete protection against various parasite strains. The major merozoite surface antigens of Babesia canis have been described as a 28-kDa membrane protein family, anchored at the surface of the merozoite. Here, we demonstrate that Bc28.1, a major member of this multigenic family, is expressed at high levels at the surface of the merozoite. This protein is also found in the parasite in vitro culture supernatants, which are the basis of effective vaccines against canine babesiosis. We defined the erythrocyte binding function of Bc28.1 and determined its high resolution solution structure using NMR spectroscopy. Surprisingly, although these proteins are thought to play a similar role in the adhesion process, the structure of Bc28.1 from B. canis appears unrelated to the previously published structure of Bd37 from B. divergens. Site-directed mutagenesis experiments also suggest that the mechanism of the interaction with the erythrocyte membrane could be different for the two proteins. The resolution of the structure of Bc28 represents a milestone for the characterization of the parasite erythrocyte binding and its interaction with the host immune system.
Organizational Affiliation:
Centre de Biochimie Structurale, CNRS UMR 5048, Université Montpellier 1 et 2, Montpellier, France.