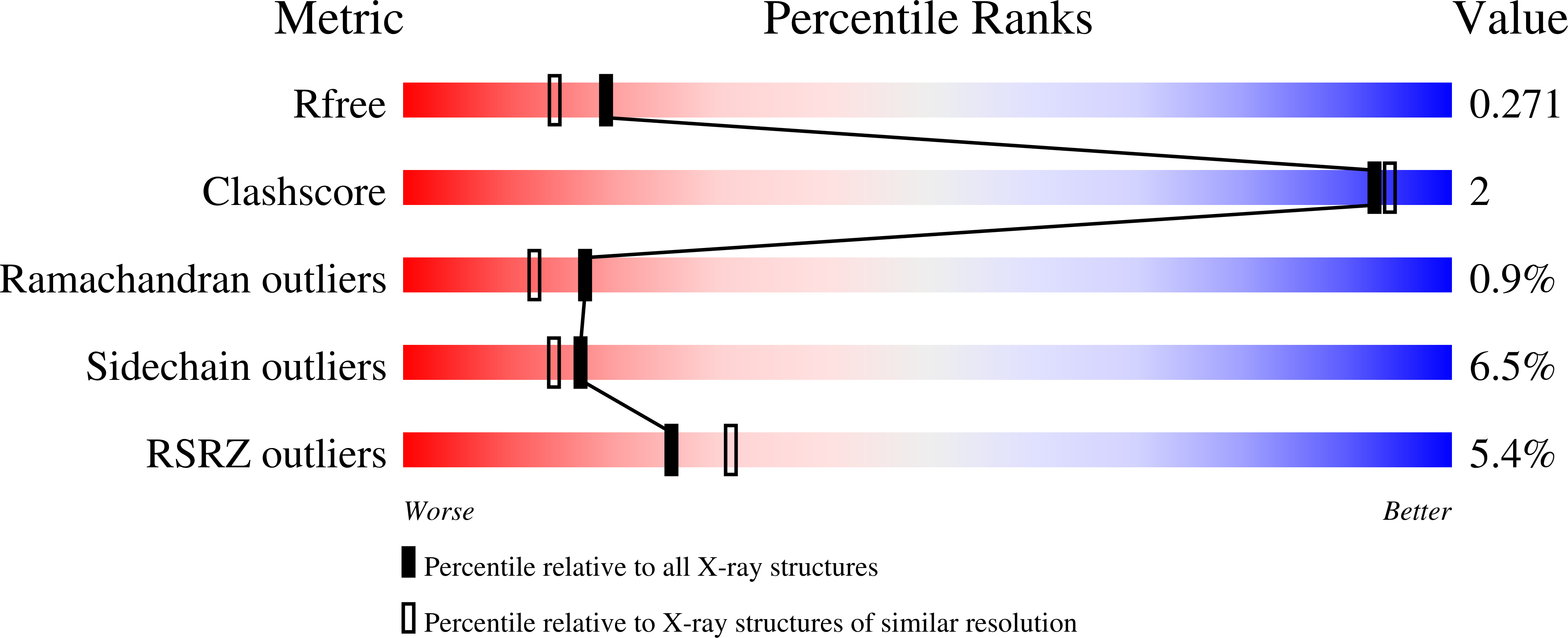
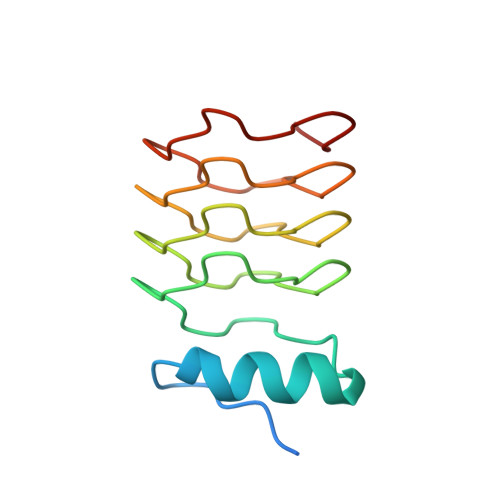
Structural Characterization of the Fusion of Two Pentapeptide Repeat Proteins, Np275 and Np276, from Nostoc Punctiforme: Resurrection of an Ancestral Protein.
Vetting, M.W., Hegde, S.S., Hazleton, K.Z., Blanchard, J.S.(2007) Protein Sci 16: 755
- PubMed: 17384236
- DOI: https://doi.org/10.1110/ps.062637707
- Primary Citation of Related Structures:
2J8I, 2J8K - PubMed Abstract:
The Nostoc punctiforme genes Np275 and Np276 are two adjacently encoded proteins of 98 and 75 amino acids in length and exhibit sequences composed of tandem pentapeptide repeats. The structures of Np275 and a fusion of Np275 and Np276 were determined to 2.1 and 1.5 A, respectively. The two Nostoc proteins fold as highly symmetric right-handed quadrilateral beta-helices similar to the mycobacterial protein MfpA implicated in fluoroquinolone resistance and DNA gyrase inhibition. The sequence composition of the intervening coding region and the ability to express a fused protein by removing the stop codon for Np275 suggests Np275 and Np276 were recently part of a larger ancestral pentapeptide repeat protein.
Organizational Affiliation:
Department of Biochemistry, Albert Einstein College of Medicine, Bronx, New York 10461, USA.