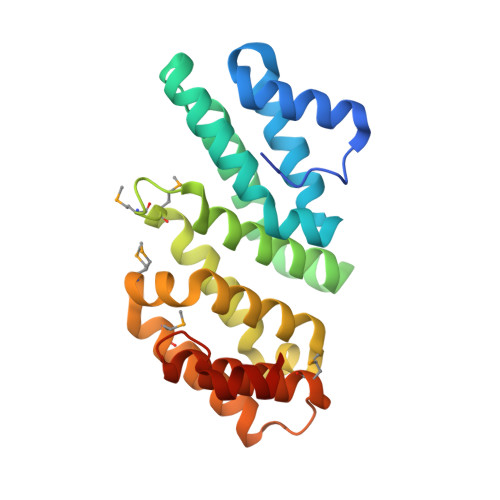
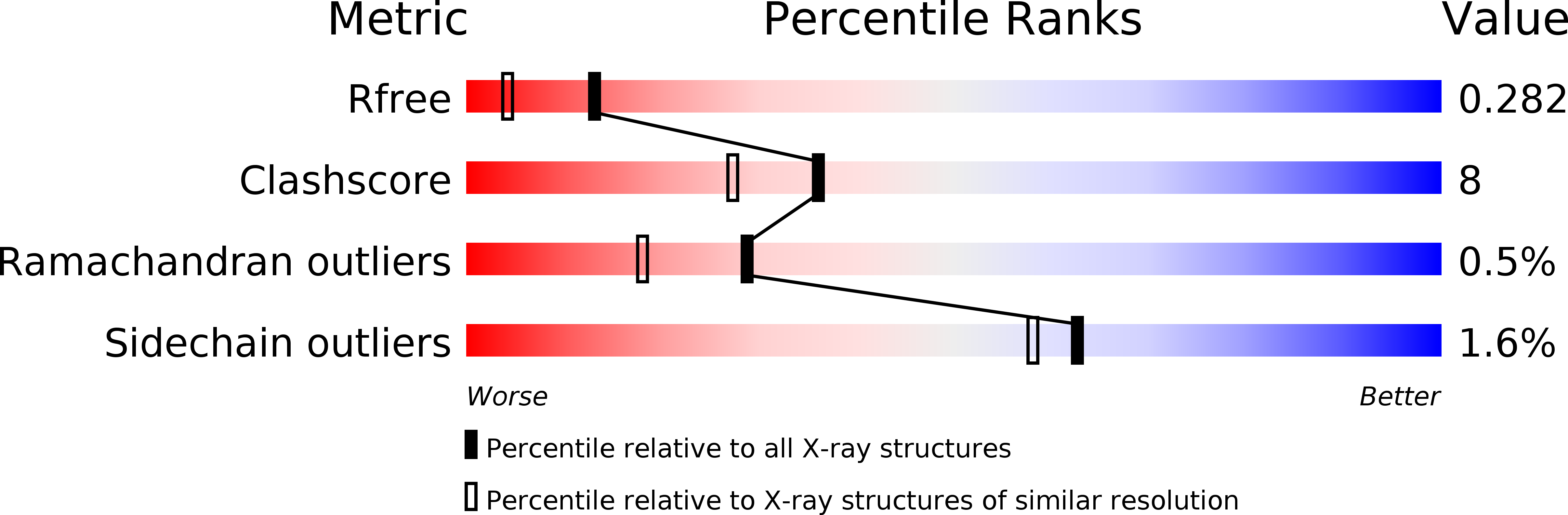Crystal structure of an eIF4G-like protein from Danio rerio.
Bae, E., Bitto, E., Bingman, C.A., McCoy, J.G., Wesenberg, G.E., Phillips, G.N.(2010) Proteins 78: 1803-1806
- PubMed: 20229607
- DOI: https://doi.org/10.1002/prot.22703
- Primary Citation of Related Structures:
2I2O
Organizational Affiliation:
Center for Eukaryotic Structural Genomics, University of Wisconsin-Madison, Madison, Wisconsin 53706, USA.