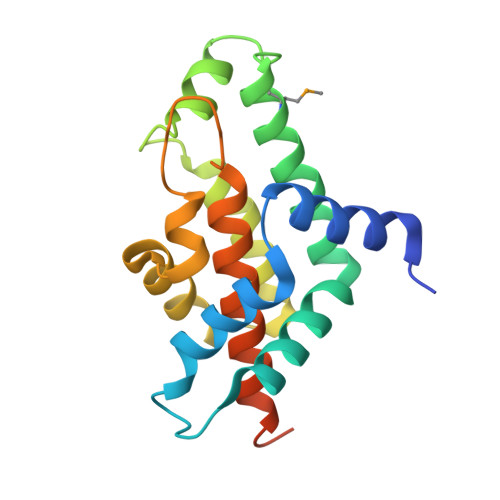
Crystal structure of phosphatidylglycerophosphatase (PGPase), a putative membrane-bound lipid phosphatase, reveals a novel binuclear metal binding site and two "proton wires".
Kumaran, D., Bonanno, J.B., Burley, S.K., Swaminathan, S.(2006) Proteins 64: 851-862
- PubMed: 16838328
- DOI: https://doi.org/10.1002/prot.21039
- Primary Citation of Related Structures:
1Y9I - PubMed Abstract:
Phosphatidylglycerophosphatase (PGPase), an enzyme involved in lipid metabolism, catalyzes formation of phosphatidylglycerol from phosphatidylglycerophosphate. Phosphatidylglycerol is a multifunctional phospholipid, found in the biological membranes of many organisms. Here, we report the crystal structure of Listeria monocytogenes PGPase at 1.8 A resolution. PGPase, an all-helical molecule, forms a homotetramer. Each protomer contains an independent active site with two metal ions, Ca(2+) and Mg(2+), forming a hetero-binuclear center located in a hydrophilic cavity near the surface of the molecule. The binuclear center, conserved ligands, metal-bound water molecules, and an Asp-His dyad form the active site. The catalytic mechanism of this enzyme is likely to proceed via binuclear metal activated nucleophilic water. The binuclear metal-binding active-site environment of this structure should provide insights into substrate binding and metal-dependent catalysis. A long channel with inter-linked linear water chains, termed "proton wires," is observed at the tetramer interface. Comparison of similar water chain structures in photosynthetic reaction centers (RCs), Cytochrome f, gramicidin, and bacteriorhodopsin, suggests that PGPase may conduct protons via proton wires.
Organizational Affiliation:
Biology Department, Brookhaven National Laboratory, Upton, New York 11973, USA.