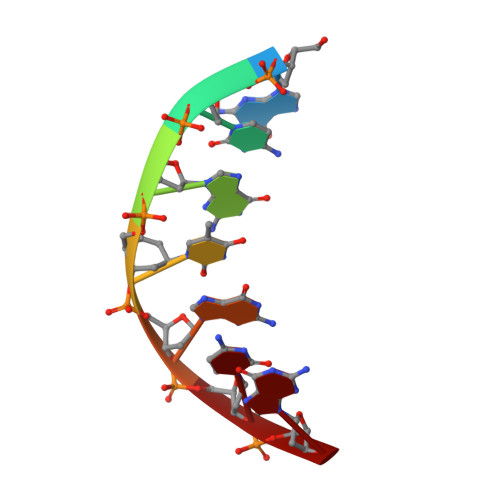
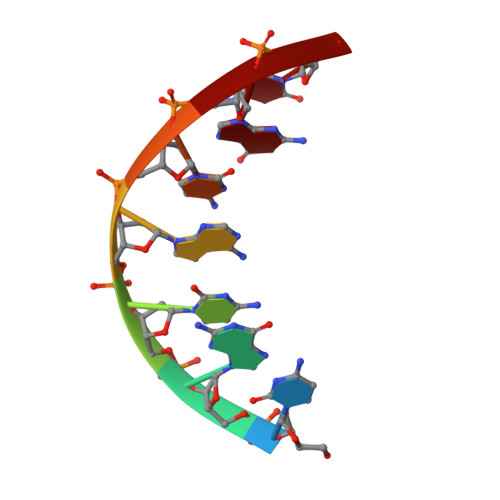
Cyclohexenyl nucleic acids: conformationally flexible oligonucleotides.
Nauwelaerts, K., Lescrinier, E., Sclep, G., Herdewijn, P.(2005) Nucleic Acids Res 33: 2452-2463
- PubMed: 15863723
- DOI: https://doi.org/10.1093/nar/gki538
- Primary Citation of Related Structures:
1U01 - PubMed Abstract:
Cyclohexenyl nucleic acid (CeNA) is a nucleic acid mimic, where the (deoxy)ribose sugar has been replaced by cyclohexenyl moieties. In order to study the conformation of cyclohexenyl nucleosides by NMR, the HexRot program was developed to calculate conformations from scalar coupling constants of cyclohexenyl compounds, analogous to the methods applied for (deoxy)ribose nucleosides. The conformational equilibria and the values of the thermodynamic parameters are very similar between a cyclohexenyl nucleoside [energy difference between 2H3 (N-type) and 2H3 (S-type) is 1.8 kJ/mol and equilibrium occurs via the eastern hemisphere with a barrier of 10.9 kJ/mol] and a natural ribose nucleoside (energy difference between N-type and S-type is 2 kJ/mol and equilibrium occurs via the eastern hemisphere with a barrier of 4-20 kJ/mol). The flexibility of the cyclohexenyl nucleoside was demonstrated by the fast equilibrium between two conformational states that was observed in a CeNA-U monomer, combined with the 2H3 conformation of the cyclohexene moiety when incorporated into a Dickerson dodecamer and the 2H3 conformation when incorporated in a d(5'-GCGT*GCG-3')/d(5'-CGCACGC-3') duplex, as determined by the NMR spectroscopy. This represents the first example of a synthetic nucleoside that adopts different conformations when incorporated in different double-stranded DNA sequences.
Organizational Affiliation:
Rega Institute for Medical Research, Laboratory for Medicinal Chemistry Minderbroedersstraat 10, B-3000 Leuven, Belgium.