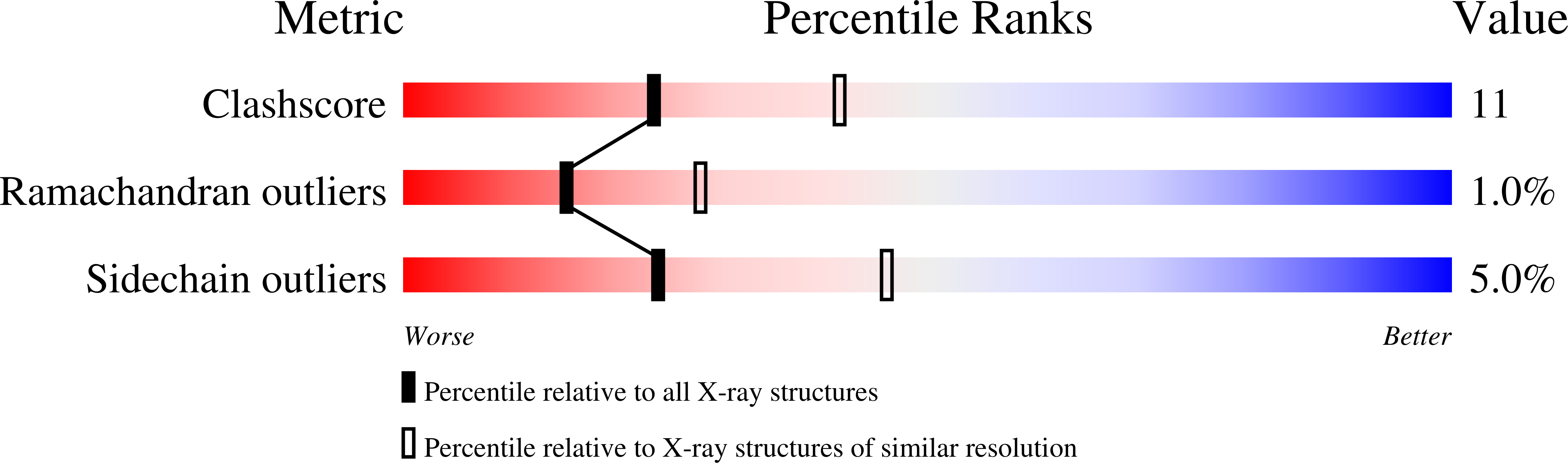Structure-based design of inhibitors specific for bacterial thymidylate synthase.
Stout, T.J., Tondi, D., Rinaldi, M., Barlocco, D., Pecorari, P., Santi, D.V., Kuntz, I.D., Stroud, R.M., Shoichet, B.K., Costi, M.P.(1999) Biochemistry 38: 1607-1617
- PubMed: 9931028
- DOI: https://doi.org/10.1021/bi9815896
- Primary Citation of Related Structures:
1TSL, 1TSM - PubMed Abstract:
Thymidylate synthase is an attractive target for antiproliferative drug design because of its key role in the synthesis of DNA. As such, the enzyme has been widely targeted for anticancer applications. In principle, TS should also be a good target for drugs used to fight infectious disease. In practice, TS is highly conserved across species, and it has proven to be difficult to develop inhibitors that are selective for microbial TS enzymes over the human enzyme. Using the structure of TS from Lactobacillus casei in complex with the nonsubstrate analogue phenolphthalein, inhibitors were designed to take advantage of features of the bacterial enzyme that differ from those of the human enzyme. Upon synthesis and testing, these inhibitors were found to be up to 40-fold selective for the bacterial enzyme over the human enzyme. The crystal structures of two of these inhibitors in complex with TS suggested the design of further compounds. Subsequent synthesis and testing showed that these second-round compounds inhibit the bacterial enzyme at sub-micromolar concentrations, while the human enzyme was not inhibited at detectable levels (selectivities of 100-1000-fold or greater). Although these inhibitors share chemical similarities, X-ray crystal structures reveal that the analogues bind to the enzyme in substantially different orientations. Site-directed mutagenesis experiments suggest that the individual inhibitors may adopt multiple configurations in their complexes with TS.
Organizational Affiliation:
Department of Biochemistry, University of California, San Francisco 94143-0448, USA.