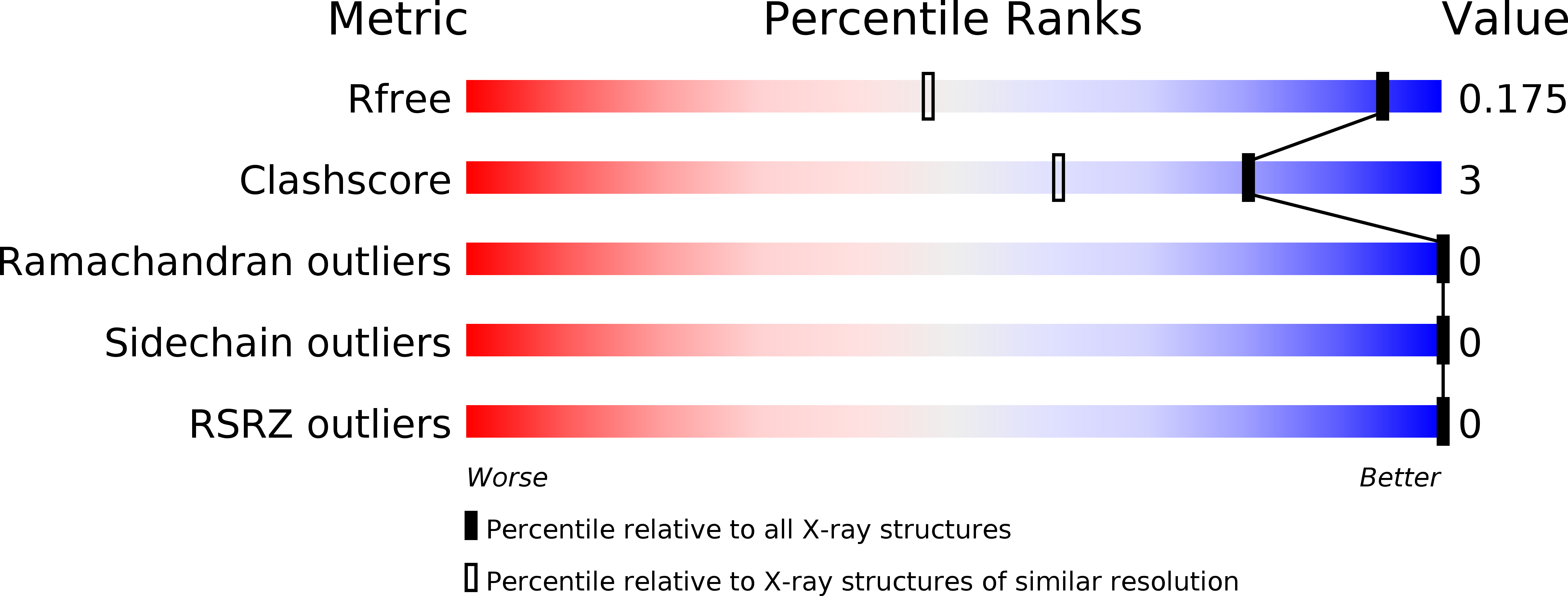
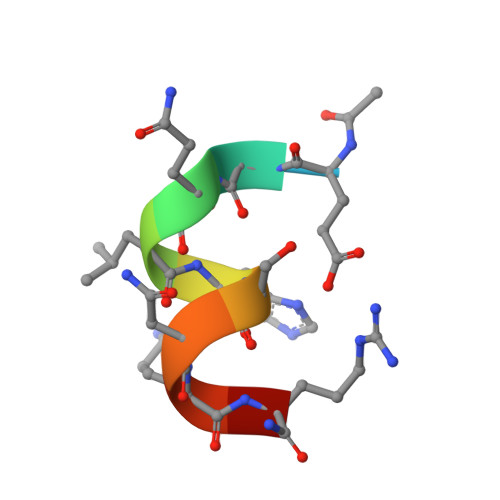
Miniaturized heme proteins: crystal structure of Co(III)-mimochrome IV.
Di Costanzo, L., Geremia, S., Randaccio, L., Nastri, F., Maglio, O., Lombardi, A., Pavone, V.(2004) J Biol Inorg Chem 9: 1017-1027
- PubMed: 15551102
- DOI: https://doi.org/10.1007/s00775-004-0600-x
- Primary Citation of Related Structures:
1PYZ - PubMed Abstract:
Protein design provides an attractive approach to test the essential features required for folding and function. Previously, we described the design and structural characterization in solution of mimochromes, a series of miniaturized metalloproteins, patterned after the F-helix of the hemoglobin beta-chain. Mimochromes consist of two medium-sized helical peptides, covalently linked to the deuteroporphyrin. CD and NMR characterization of the prototype, mimochrome I, revealed that the overall structure conforms well to the design. However, formation of Delta and Lambda diastereomers was observed. To overcome the problem of diastereomer formation, we re-designed mimochrome I, by engineering intramolecular, interchain interactions. The resulting model was mimochrome IV: the solution structural characterization showed the presence of the Lambda isomer as a unique form. To examine the extent to which the stereochemical stability and uniqueness of mimochrome IV was retained in the solid state, the crystal structure of Co(III)-mimochrome IV was solved by X-ray diffraction, and compared to the solution structure of the same derivative. Co(III)-mimochrome IV structures, both in solution and in the solid state, are characterized by the following common features: a bis-His axial coordination, a Lambda configuration around the metal ion, and a predominant helical conformation of the peptide chains. However, in the crystal structure, intrachain Glu1-Arg9 ion pairs are preferred over the designed, and experimentally found in solution, interchain interactions. This ion pairing switch may be related to strong packing interactions.
Organizational Affiliation:
Centre of Excellence in Biocrystallography, Department of Chemical Science, University of Trieste, Via L. Giorgieri 1, 34127, Trieste, Italy.