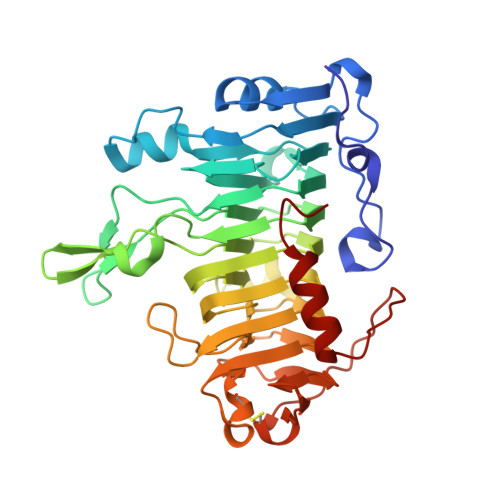
Effect of mutations in the T1.5 loop of pectate lyase A from Erwinia chrysanthemi EC16.
Dehdashti, S.J., Doan, C.N., Chao, K.L., Yoder, M.D.(2003) Acta Crystallogr D Biol Crystallogr 59: 1339-1342
- PubMed: 12832805
- DOI: https://doi.org/10.1107/s0907444903011491
- Primary Citation of Related Structures:
1OOC, 1PE9 - PubMed Abstract:
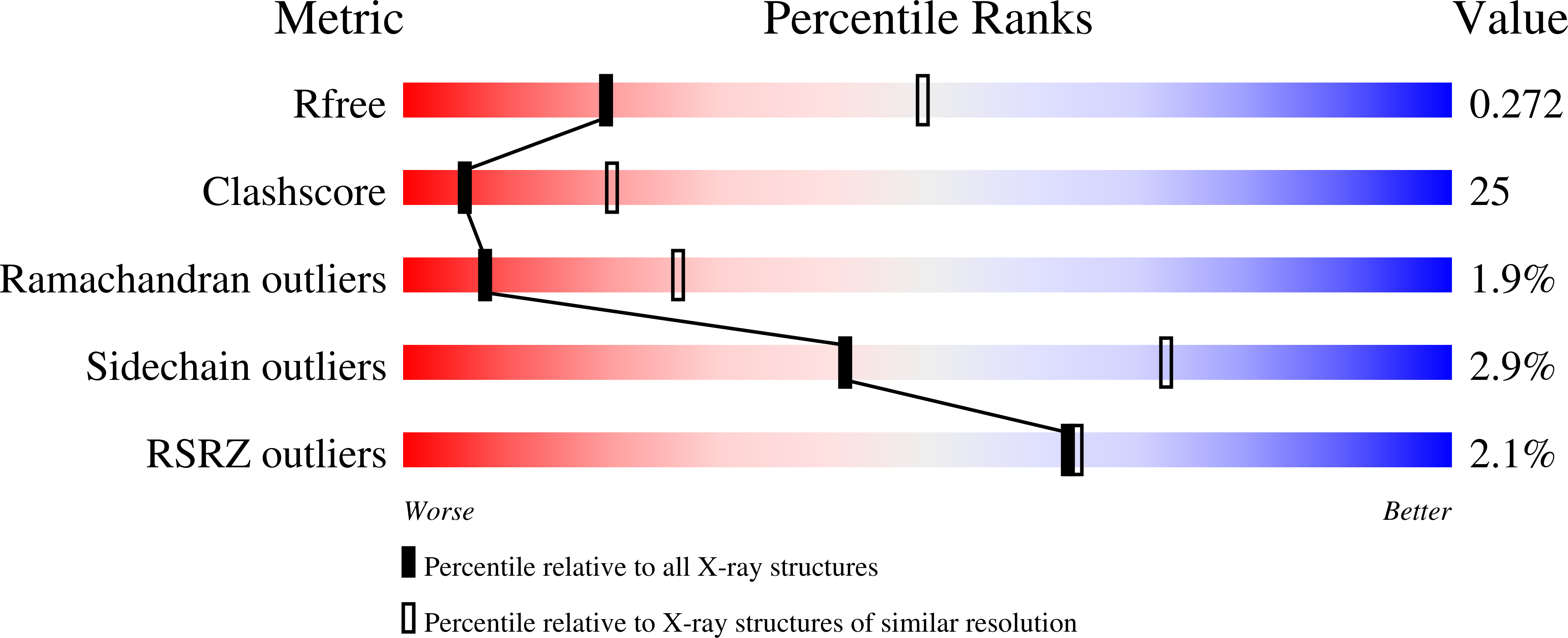
Pectate lyase A (PelA) is a pectate-degrading enzyme secreted by plant pathogens. PelA from Erwinia chrysanthemi has 61% amino-acid identity and a conserved structural similarity to pectate lyase E (PelE). Although similar in structure and sequence, the enzymatic characteristics of PelA differ from those for PelE. A structural alignment of PelA and PelE reveals differences in the T1.5 loop. The sequence of the T1.5 loop in PelA was mutated to the homologous sequence in PelE. The crystal structure of the PelA T1.5 mutant has been solved to 1.6 and 2.9 A resolution. The enzymatic and structural properties of the T1.5 mutant are discussed.
Organizational Affiliation:
University of Missouri Kansas City, School of Biological Sciences, 5100 Rockhill Road, Kansas City, Missouri 64111-2499, USA.