Crystal Structure of a Putative Methyltransferase from Mycobacterium tuberculosis: Misannotation of a Genome Clarified by Protein Structural Analysis
Johnston, J.M., Arcus, V.L., Morton, C.J., Parker, M.W., Baker, E.N.(2003) J Bacteriol 185: 4057-4065
- PubMed: 12837779
- DOI: https://doi.org/10.1128/JB.185.14.4057-4065.2003
- Primary Citation of Related Structures:
1NXJ - PubMed Abstract:
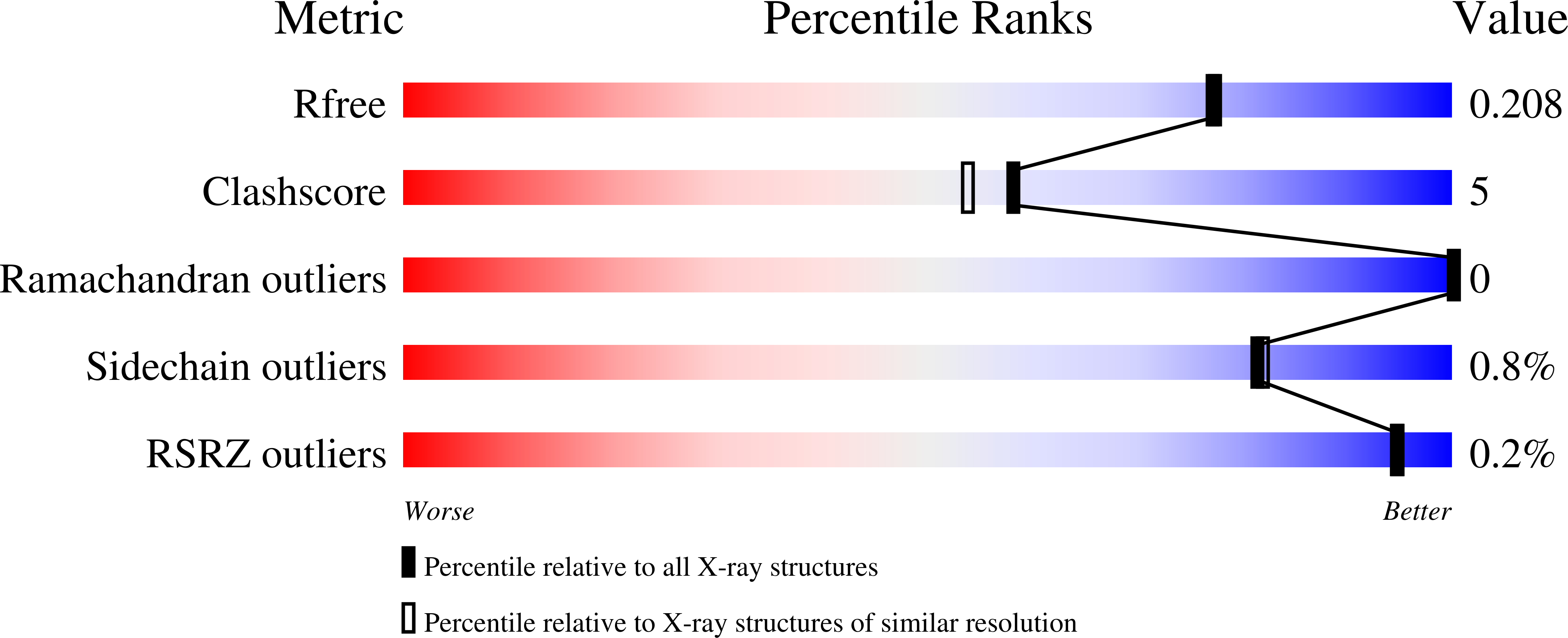
Bioinformatic analyses of whole genome sequences highlight the problem of identifying the biochemical and cellular functions of many gene products that are at present uncharacterized. The open reading frame Rv3853 from Mycobacterium tuberculosis has been annotated as menG and assumed to encode an S-adenosylmethionine (SAM)-dependent methyltransferase that catalyzes the final step in menaquinone biosynthesis. The Rv3853 gene product has been expressed, refolded, purified, and crystallized in the context of a structural genomics program. Its crystal structure has been determined by isomorphous replacement and refined at 1.9 A resolution to an R factor of 19.0% and R(free) of 22.0%. The structure strongly suggests that this protein is not a SAM-dependent methyltransferase and that the gene has been misannotated in this and other genomes that contain homologs. The protein forms a tightly associated, disk-like trimer. The monomer fold is unlike that of any known SAM-dependent methyltransferase, most closely resembling the phosphohistidine domains of several phosphotransfer systems. Attempts to bind cofactor and substrate molecules have been unsuccessful, but two adventitiously bound small-molecule ligands, modeled as tartrate and glyoxalate, are present on each monomer. These may point to biologically relevant binding sites but do not suggest a function. In silico screening indicates a range of ligands that could occupy these and other sites. The nature of these ligands, coupled with the location of binding sites on the trimer, suggests that proteins of the Rv3853 family, which are distributed throughout microbial and plant species, may be part of a larger assembly binding to nucleic acids or proteins.
Organizational Affiliation:
School of Biological Sciences, University of Auckland, Auckland, New Zealand. St. Vincent's Institute of Medical Research, Fitzroy, Victoria 3065, Australia.