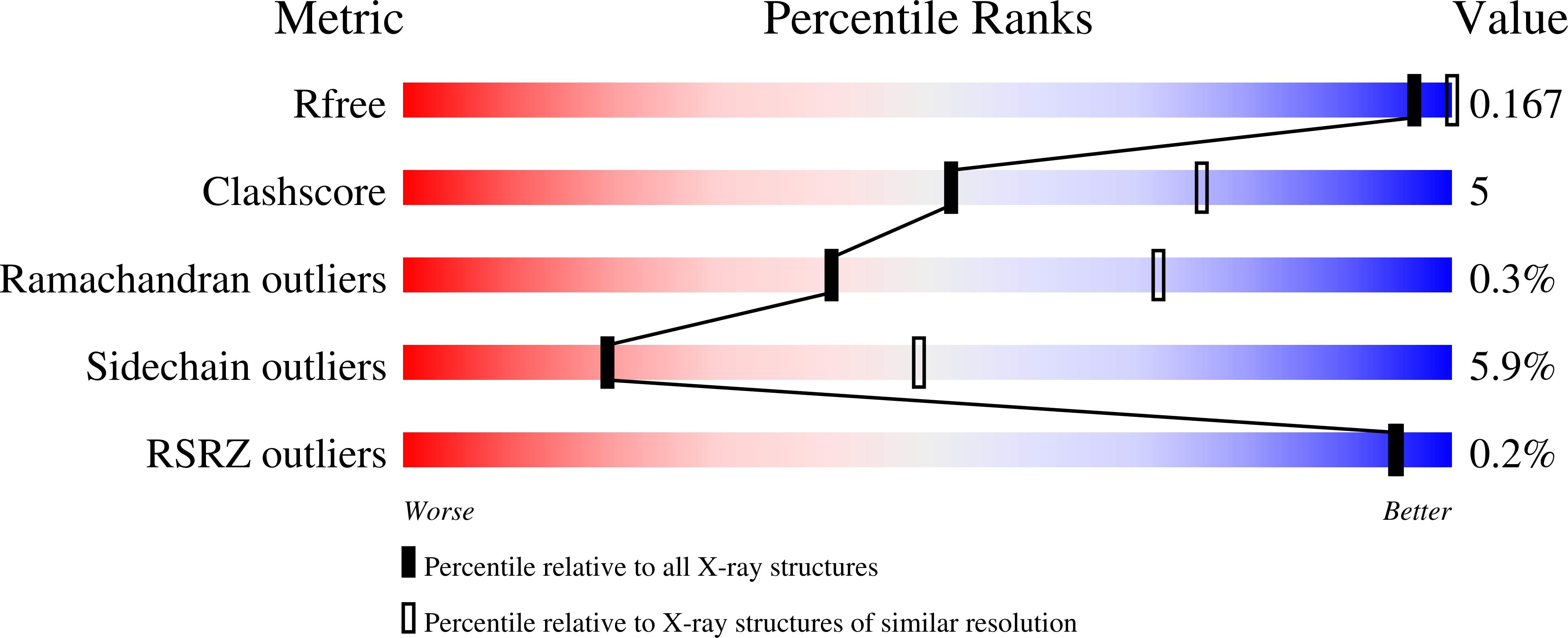
Structure of maltoporin from Salmonella typhimurium ligated with a nitrophenyl-maltotrioside.
Meyer, J.E., Hofnung, M., Schulz, G.E.(1997) J Mol Biol 266: 761-775
- PubMed: 9102468
- DOI: https://doi.org/10.1006/jmbi.1996.0823
- Primary Citation of Related Structures:
1MPR, 2MPR - PubMed Abstract:
The maltodextrin-specific (malto-)porin from Salmonella typhimurium has been crystallized. Its three-dimensional structure was determined at 2.4 A resolution (1 A = 0.1 nm). A comparison with the structure of the homologous porin from Escherichia coli as well as with the sequences of other related porins showed that there are regions of appreciable sequence and structure variability, despite close overall similarity. The maltoporin structure was analyzed with a bound nitrophenyl-maltotrioside as well as without ligand. Maltotrioside binding had a negligible effect on the polypeptide structure. It binds at the pore eyelet assuming a conformation close to the natural amylose helix.
Organizational Affiliation:
Institut für Organische Chemie und Biochemie Albert-Ludwigs-Universität, Freiburg im Breisgau Germany.