Toward engineering the stability and hemin binding properties of microsomal cytochromes b5 into rat outer mitochondrial cytochrome b5: Examining the influence of residues 25 and 71.
Cowley, A.B., Altuve, A., Kuchment, O., Terzyan, S., Zhang, X.C., Rivera, M., Benson, D.(2002) Biochemistry 41: 11566-11581
- PubMed: 12269800
- DOI: https://doi.org/10.1021/bi026005l
- Primary Citation of Related Structures:
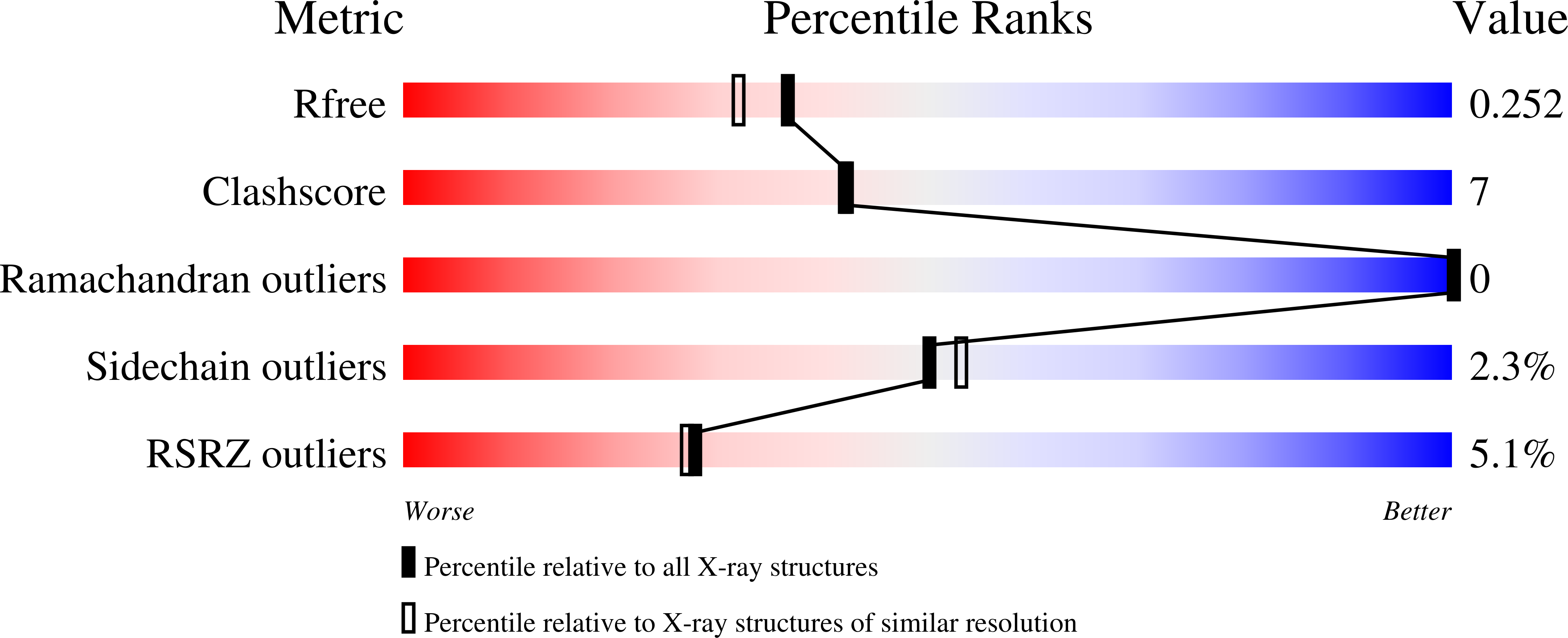
1LJ0 - PubMed Abstract:
As part of a larger effort to engineer the stability and hemin-binding properties of microsomal (Mc) cytochromes b(5) into rat liver outer mitochondrial membrane (OM) cytochrome (cyt) b(5), several mutants of rat OM cyt b(5) were prepared to study the effect of gradual and complete elimination of two extended hydrophobic networks, which are present in the structure of the mitochondrial protein and are absent in the structure of mammalian Mc cytochromes b(5). One of the hydrophobic networks, identified in a previous study [Altuve, A., Silchenko, S., Lee, K.-H., Kuczera, K., Terzyan, S., Zhang, X., Benson, D. R., and Rivera, M. (2001) Biochemistry 40, 9469-9483], encompasses the side chains of Ala-18, Ile-32, Leu-36, and Leu-47, whereas a second hydrophobic network, identified as part of this work, encompasses the side chains of Ile-25, Phe-58, Leu-71, and the heme. The X-ray structure of the A18S/I25L/I32L/L47R/L71S quintuple mutant of rat OM cyt b(5) demonstrates that both hydrophobic networks have been eliminated and that the corresponding structural elements of the Mc isoform have been introduced. The stability of the rat OM mutant proteins studied was found to decrease in the order wild type > I25L > A18S/I32L/L47R > L71S > A18S/I32L/L47R/L71S > 18S/I25L/I32L/L47R/L71S, indicating that the two hydrophobic networks do indeed contribute to the high stability of rat OM cyt b(5) relative to the bovine Mc isoform. Surprisingly, the quintuple mutant of rat OM cyt b(5) is less stable than bovine Mc cyt b(5), even though the former exhibits significantly slower rates of hemin release and hemin reorientation at pH 7.0. However, at pH 5.0 the bovine Mc and rat OM quintuple mutant proteins release hemin at comparable rates, suggesting that one or both of the His axial ligands in the rat OM protein are more resistant to protonation under physiological conditions. Results obtained from chemical denaturation experiments conducted with the apoproteins demonstrated that mutants containing L71S are significantly less stable than bovine Mc apocyt b(5), strongly suggesting that Leu-71 plays a pivotal role in the stabilization of rat OM apocyt b(5), presumably via hydrophobic interactions with Ile-25 and Phe-58. Because comparable interactions are absent in bovine Mc apocyt b(5), which contains Ser at position 71, it must resort to different interactions to stabilize its fold, thus highlighting yet another difference between rat OM and bovine Mc cyt b(5). During the course of these investigations we also discovered that rat OM cyt b(5) can be made to strongly favor hemin orientational isomer A (I32L) or isomer B (L71S) with a single point mutation and that release of hemin orientational isomers A and B can be kinetically resolved in certain rat OM mutants.
Organizational Affiliation:
Department of Chemistry, University of Kansas, Lawrence, KS 66045-7582, USA.