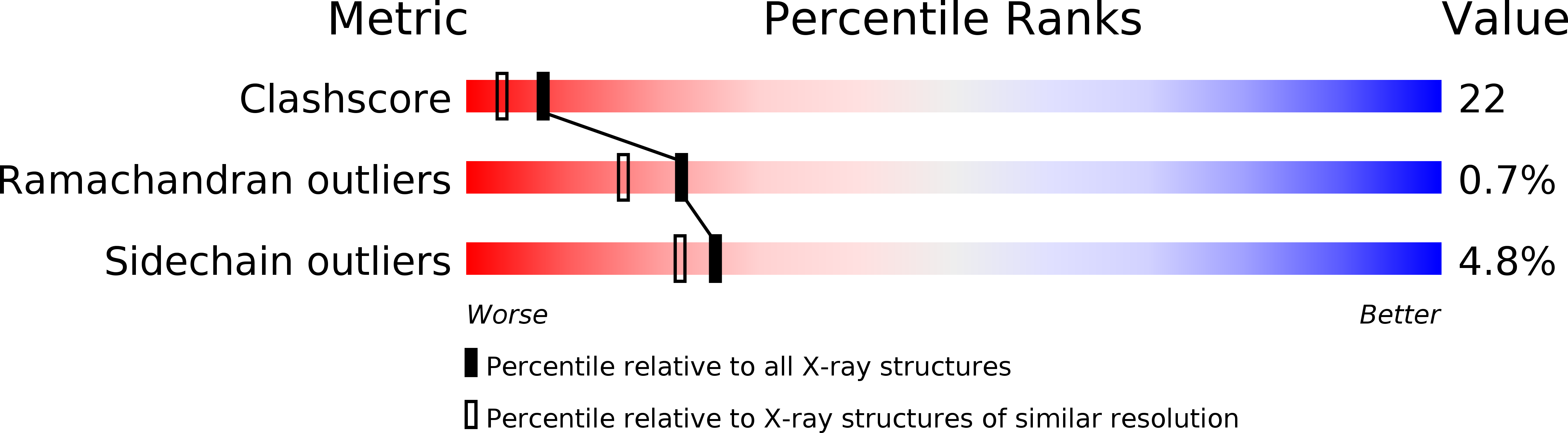Crystal structure of the ADP-dependent glucokinase from Pyrococcus horikoshii at 2.0-A resolution: a large conformational change in ADP-dependent glucokinase
Tsuge, H., Sakuraba, H., Kobe, T., Kujime, A., Katunuma, N., Ohshima, T.(2002) Protein Sci 11: 2456-2463
- PubMed: 12237466
- DOI: https://doi.org/10.1110/ps.0215602
- Primary Citation of Related Structures:
1L2L - PubMed Abstract:
Although ATP is the most common phosphoryl group donor for kinases, some kinases from certain hyperthermophilic archaea such as Pyrococcus horikoshii and Thermococcus litoralis use ADP as the phosphoryl donor. Those are ADP-dependent glucokinases (ADPGK) and phosphofructokinases in their glycolytic pathway. Here, we succeeded in gene cloning the ADPGK from P. horikoshii OT3 (phGK) in Escherichia coli,and in easy preparation of the enzyme, crystallization, and the structure determination of the apo enzyme. Recently, the three-dimensional structure of the ADPGK from T. litoralis (tlGK) in a complex with ADP was reported. The overall structure of two homologous enzymes (56.7%) was basically similar: This means that they consisted of large alpha/beta-domains and small domains. However, a marked adjustment of the two domains, which is a 10-A translation and a 20 degrees rotation from the conserved GG sequence located at the center of the hinge, was observed between the apo-phGK and ADP-tlGK structures. The ADP-binding loop (430-439) was disordered in the apo form. It is suggested that a large conformational change takes place during the enzymatic reaction.
Organizational Affiliation:
Institute for Health Sciences, Tokushima Bunri University, Yamashiro-cho, Japan.