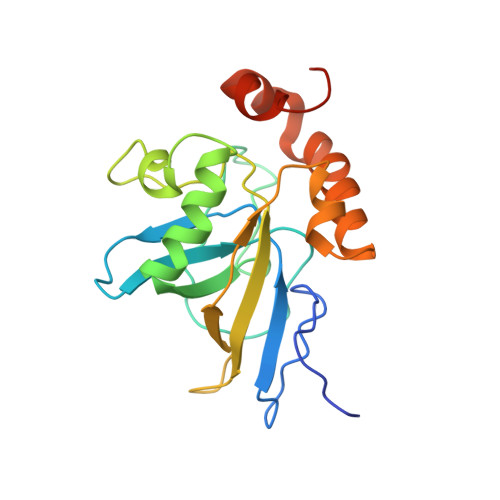
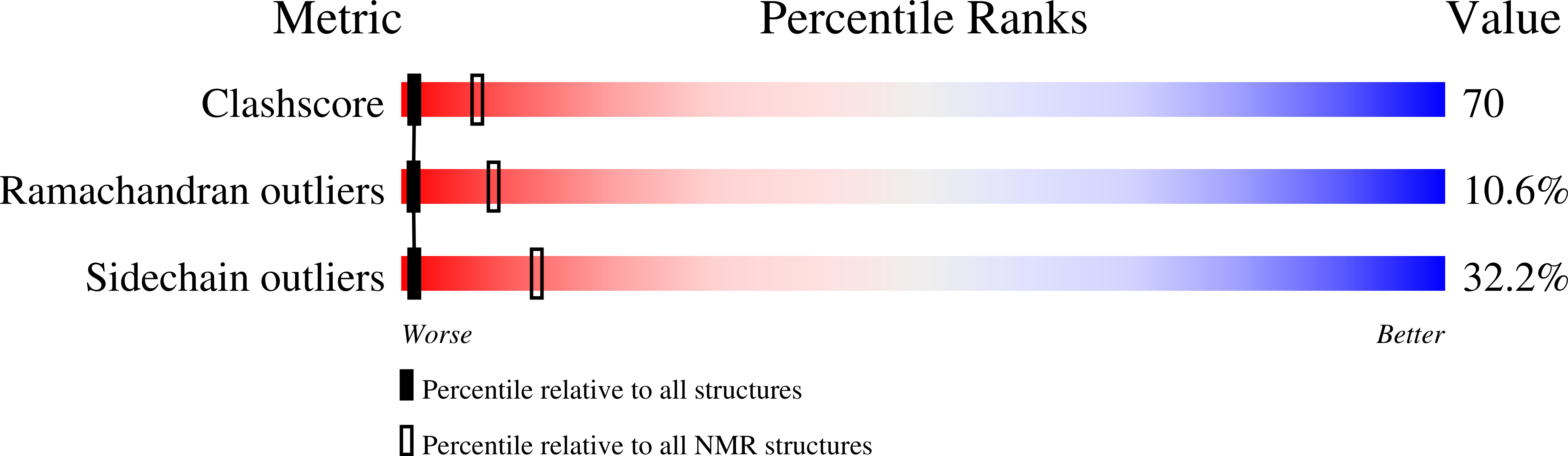Conserved structure for single-stranded telomeric DNA recognition.
Mitton-Fry, R.M., Anderson, E.M., Hughes, T.R., Lundblad, V., Wuttke, D.S.(2002) Science 296: 145-147
- PubMed: 11935027
- DOI: https://doi.org/10.1126/science.1068799
- Primary Citation of Related Structures:
1KXL - PubMed Abstract:
The essential Cdc13 protein in the yeast Saccharomyces cerevisiae is a single-stranded telomeric DNA binding protein required for chromosome end protection and telomere replication. Here we report the solution structure of the Cdc13 DNA binding domain in complex with telomeric DNA. The structure reveals the use of a single OB (oligonucleotide/oligosaccharide binding) fold augmented by an unusually large loop for DNA recognition. This OB fold is structurally similar to OB folds found in the ciliated protozoan telomere end-binding protein, although no sequence similarity is apparent between them. The common usage of an OB fold for telomeric DNA interaction demonstrates conservation of end-protection mechanisms among eukaryotes.
Organizational Affiliation:
Department of Chemistry and Biochemistry, University of Colorado, Boulder, CO 80309, USA.