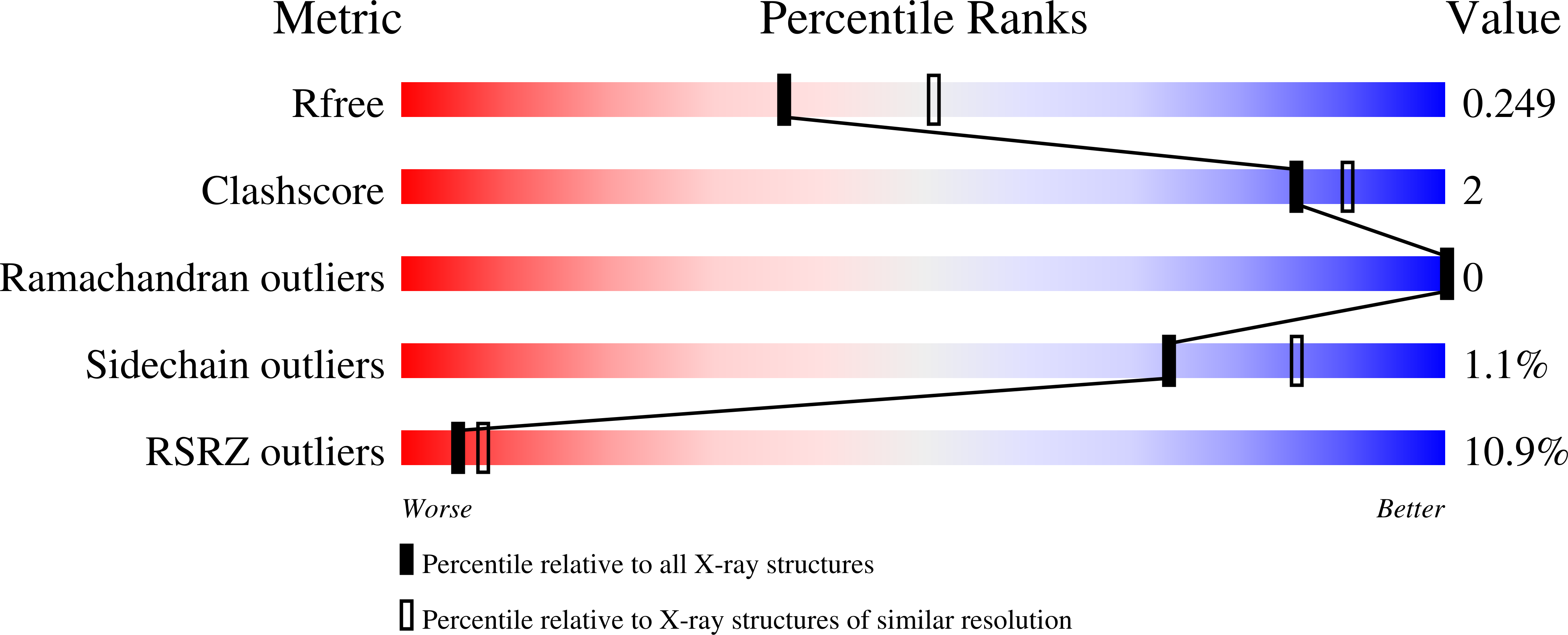
Cosolvent-induced transformation of a death domain tertiary structure
Xiao, T., Gardner, K.H., Sprang, S.R.(2002) Proc Natl Acad Sci U S A 99: 11151-11156
- PubMed: 12177432
- DOI: https://doi.org/10.1073/pnas.172188399
- Primary Citation of Related Structures:
1IK7 - PubMed Abstract:
The death domain (DD) of the protein kinase Pelle adopts a six-helix bundle fold in the crystal structure of the complex with its dimerization partner, Tube-DD. However, in crystals obtained from a solution of 45% 2-methyl-2,4-pentanediol (MPD), the C-terminal half of Pelle-DD folds into a single helix, and the N-terminal half of the molecule is disordered. The helical segment forms an antiparallel dimer with the corresponding helix of a symmetry-related molecule, and together they form extensive lattice interactions similar in number, composition, and buried surface to those in the six-helix bundle of the native fold. Secondary structure analysis by heteronuclear nuclear magnetic resonance spectroscopy (NMR) demonstrates that Pelle-DD adopts a six-helix bundle fold in aqueous solution. The fold is perturbed by MPD, with the largest chemical shift changes in one helix and two loop regions that encompass the Tube-DD binding site. Pelle-DD is stable to urea denaturation with a folding free energy of 7.9 kcal/mol at 25 degrees C but is destabilized, with loss of urea binding sites, in the presence of MPD. The data are consistent with a cosolvent denaturation model in which MPD denatures the N terminus of Pelle-DD but induces the C terminus to form a more compact structure and aggregate. A similar perturbation in vivo might occur at the plasma membrane and could have consequences for Pelle-mediated regulation. Generally, crystallographers should be aware that high concentrations of MPD or related cosolvents can alter the tertiary structure of susceptible proteins.
Organizational Affiliation:
The Howard Hughes Medical Institute and Department of Biochemistry, University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX 75390-9050, USA.