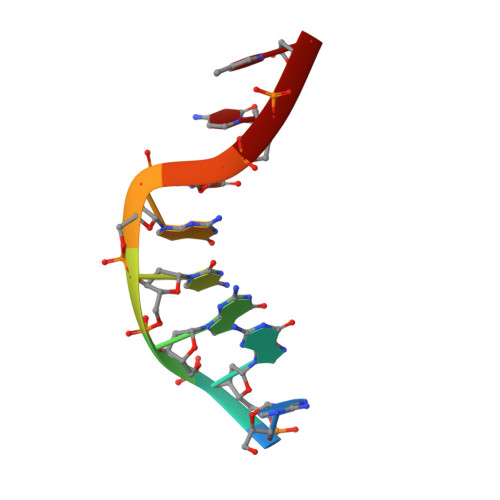
Structural studies of an oligodeoxynucleotide containing a trimethylene interstrand cross-link in a 5'-(CpG) motif: model of a malondialdehyde cross-link.
Dooley, P.A., Tsarouhtsis, D., Korbel, G.A., Nechev, L.V., Shearer, J., Zegar, I.S., Harris, C.M., Stone, M.P., Harris, T.M.(2001) J Am Chem Soc 123: 1730-1739
- PubMed: 11456774
- DOI: https://doi.org/10.1021/ja003163w
- Primary Citation of Related Structures:
1HZ2 - PubMed Abstract:
Malondialdehyde (MDA), a known mutagen and suspected carcinogen, is a product of lipid peroxidation and byproduct of eicosanoid biosynthesis. MDA can react with DNA to generate potentially mutagenic adducts on adenine, cytosine, and particularly guanine. In addition, repair-dependent frame shift mutations in a GCGCGC region of Salmonella typhimurium hisD3052 have been attributed to formation of interstrand cross-links (Mukai, F. H. and Goldstein, B. D. Science 1976, 191, 868--869). The cross-linked species is unstable and has never been characterized but has been postulated to be a bis-imino linkage between N(2) positions of guanines. An analogous linkage has now been investigated as a stable surrogate using the self-complementary oligodeoxynucleotide sequence 5'-d(AGGCG*CCT)(2,) in which G* represents guanines linked via a trimethylene chain between N(2) positions. The solution structure, obtained by NMR spectroscopy and molecular dynamics using a simulated annealing protocol, revealed the cross-link only minimally distorts duplex structure in the region of the cross-link. The tether is accommodated by partially unwinding the duplex at the lesion site to produce a bulge and tipping the guanine residues; the two guanines and the tether attain a nearly planar conformation. This distortion did not result in significant bending of the DNA, a result which was confirmed by gel electrophoresis studies of multimers of a 21-mer duplex containing the cross-link.
Organizational Affiliation:
Department of Chemistry, Vanderbilt University, Nashville, Tennessee 37235, USA.