Crystallization and Preliminary X-Ray Analysis of Two Ph-Dependent Forms of Cytochrome C2 from Rhodopseudomonas Palustris
Garau, G., Geremia, S., Randaccio, L., Vaccari, L., Viezzoli, M.S.(2000) Acta Crystallogr D Biol Crystallogr 56: 1699
- PubMed: 11092951
- DOI: https://doi.org/10.1107/s0907444900013573
- Primary Citation of Related Structures:
1HH7 - PubMed Abstract:
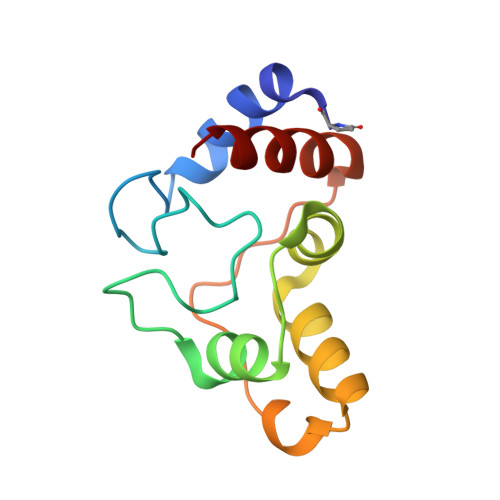
Cytochrome c(2) from Rhodopseudomonas palustris has been crystallized in two different crystal forms: a monoclinic form I at pH 4.4 from both reduced and oxidized protein solution and a trigonal form II at pH 9.0 from reduced protein solution. Complete 1. 7 and 1.4 A resolution data sets were collected from the oxidized form I and from the form II, respectively. The preliminary structures show an important change in the iron coordination environment in the trigonal form obtained at basic pH arising from the substitution of the Met ligand by an ammonia molecule.
Organizational Affiliation:
Department of Chemical Sciences, University of Trieste, Via L. Giorgieri 1, I-34127 Trieste, Italy.