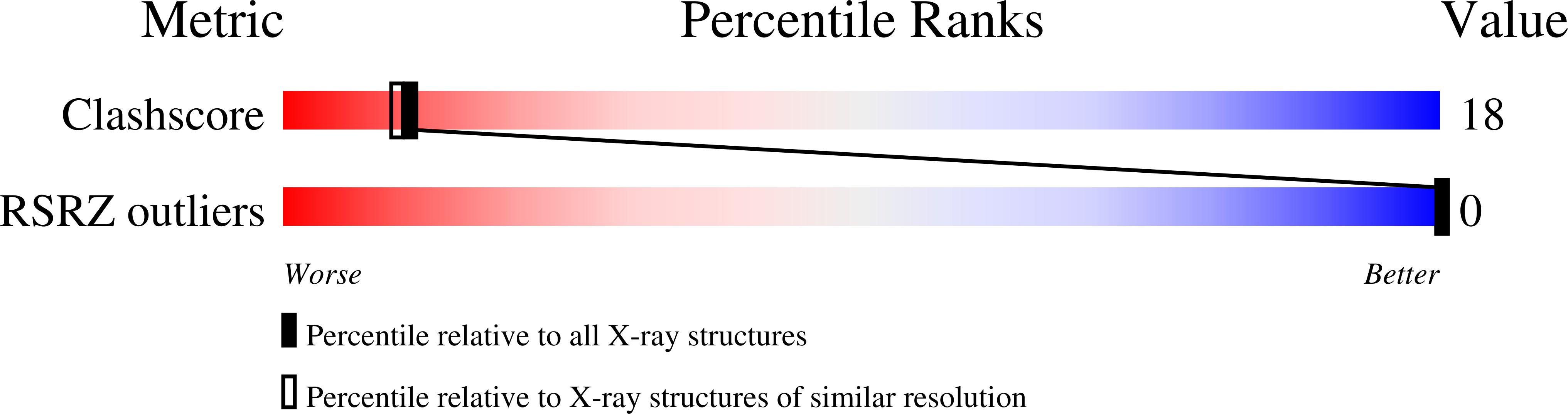The crystal structures of psoralen cross-linked DNAs: drug-dependent formation of Holliday junctions.
Eichman, B.F., Mooers, B.H., Alberti, M., Hearst, J.E., Ho, P.S.(2001) J Mol Biol 308: 15-26
- PubMed: 11302703
- DOI: https://doi.org/10.1006/jmbi.2001.4567
- Primary Citation of Related Structures:
1FHY, 1FHZ - PubMed Abstract:
The single-crystal structures are presented for two DNA sequences with the thymine bases covalently cross-linked across the complementary strands by 4'-hydroxymethyl-4,5',8-trimethylpsoralen (HMT). The HMT-adduct of d(CCGCTAGCGG) forms a psoralen-induced Holliday junction, showing for the first time the effect of this important class of chemotheraputics on the structure of the recombination intermediate. In contrast, HMT-d(CCGGTACCGG) forms a sequence-dependent junction. In both structures, the DNA duplex is highly distorted at the thymine base linked to the six-member pyrone ring of the drug. The psoralen cross-link defines the intramolecular interactions of the drug-induced junction, while the sequence-dependent structure is nearly identical to the native Holliday junction of d(CCGGTACCGG) alone. The two structures contrast the effects of drug- and sequence-dependent interactions on the structure of a Holliday junction, suggesting a role for psoralen in the mechanism to initiate repair of psoralen-lesions in mammalian DNA.
Organizational Affiliation:
Department of Biochemistry and Biophysics, ALS 2011, Oregon State University, Corvallis, OR 97331, USA.