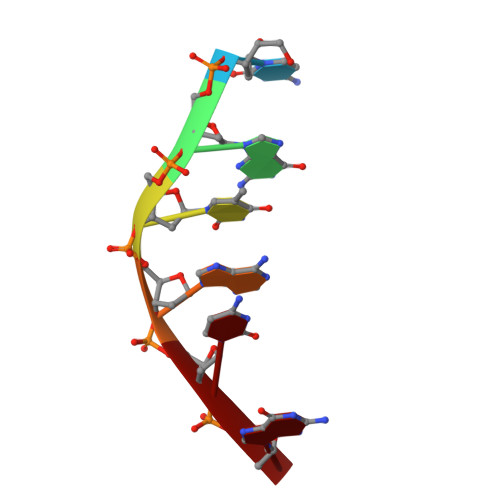
Interactions between an anthracycline antibiotic and DNA: molecular structure of daunomycin complexed to d(CpGpTpApCpG) at 1.2-A resolution.
Wang, A.H., Ughetto, G., Quigley, G.J., Rich, A.(1987) Biochemistry 26: 1152-1163
- PubMed: 3567161
- DOI: https://doi.org/10.1021/bi00378a025
- Primary Citation of Related Structures:
1D11 - PubMed Abstract:
The crystal structure of a daunomycin-d(CGTACG) complex has been solved by X-ray diffraction analysis and refined to a final R factor of 0.175 at 1.2-A resolution. The crystals are in a tetragonal crystal system with space group P4(1)2(1)2 and cell dimensions of a = b = 27.86 A and c = 52.72 A. The self-complementary DNA forms a six base pair right-handed double helix with two daunomycin molecules intercalated in the d(CpG) sequences at either end of the helix. Daunomycin in the complex has a conformation different from that of daunomycin alone. The daunomycin aglycon chromophore is oriented at right angles to the long dimension of the DNA base pairs, and the cyclohexene ring A rests in the minor groove of the double helix. Substituents on this ring have hydrogen-bonding interactions to the base pairs above and below the intercalation site. O9 hydroxyl group of the daunomycin forms two hydrogen bonds with N3 and N2 of an adjacent guanine base. Two bridging water molecules between the drug and DNA stabilize the complex in the minor groove. In the major groove, a hydrated sodium ion is coordinated to N7 of the terminal guanine and the O4 and O5 of daunomycin with a distorted octahedral geometry. The amino sugar lies in the minor groove without bonding to the DNA. The DNA double helix is distorted with an asymmetrical rearrangement of the backbone conformation surrounding the intercalator drug. The sugar puckers are C1,C2'-endo, G2,C1'-endo, C11,C1'-endo, and G12,C3'-exo. Only the C1 residue has a normal anti-glycosyl torsion angle (chi = -154 degrees), while the other three residues are all in the high anti range (average chi = -86 degrees). This structure allows us to identify three principal functional components of anthracycline antibiotics: the intercalator (rings B-D), the anchoring functions associated with ring A, and the amino sugar. The structure-function relationships of daunomycin binding to DNA as well as other related anticancer drugs are discussed.