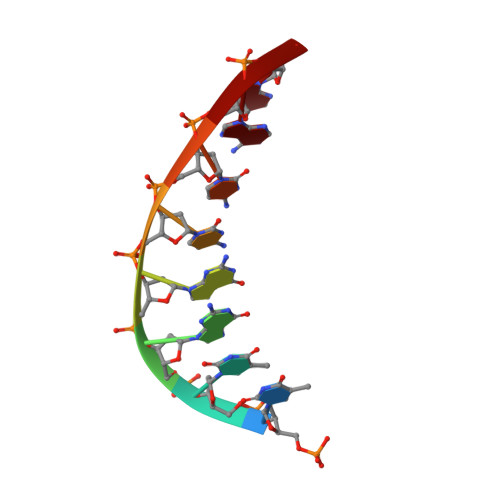
Solution structure of the novel antitumor drug UCH9 complexed with d(TTGGCCAA)2 as determined by NMR.
Katahira, R., Katahira, M., Yamashita, Y., Ogawa, H., Kyogoku, Y., Yoshida, M.(1998) Nucleic Acids Res 26: 744-755
- PubMed: 9443966
- DOI: https://doi.org/10.1093/nar/26.3.744
- Primary Citation of Related Structures:
1CP8 - PubMed Abstract:
Aureolic acid group compounds, such as chromomycin A3(CHM) and mithramycin (MIT), are known as antitumor drugs. Recently we isolated a novel aureolic acid group antitumor drug, UCH9, from Streptomyces sp. The chemical structure of UCH9 is unique in that mono- (A ring) and tetrasaccharide (B-E rings) segments and a longer hydrophobic sidechain are attached to the chromophore, while di- and trisaccharide segments and a methyl group are attached to it in the cases of CHM and MIT. It has been shown by two-dimensional agarose gel electrophoresis that the three drugs cause DNA unwinding, UCH9 causing less than the others. A photo-CIDNP experiment has revealed that UCH9 binds to the minor groove of DNA. The structure of the UCH9-d(TTGGCCAA)2 complex has been determined by 1H NMR and simulated annealing calculations. The obtained structure indicates that UCH9 binds as a dimer to the minor groove of d(TTGGCCAA)2, like CHM and MIT, but that the structural change in DNA induced on binding of UCH9 is moderate in comparison with those on binding of the other two drugs. It turns out that the dimer structure of UCH9, stabilized presumably through a hydrophobic interaction involving the A, D and E rings and the hydrophobic sidechain is different from that of CHM and thus DNA can interact with UCH9 in the minor groove with a moderate structural change.
Organizational Affiliation:
Tokyo Research Laboratories, Kyowa Hakko Kogyo Co. Ltd, 3-6-6 Asahimachi, Machida-shi, Tokyo 194, Japan.