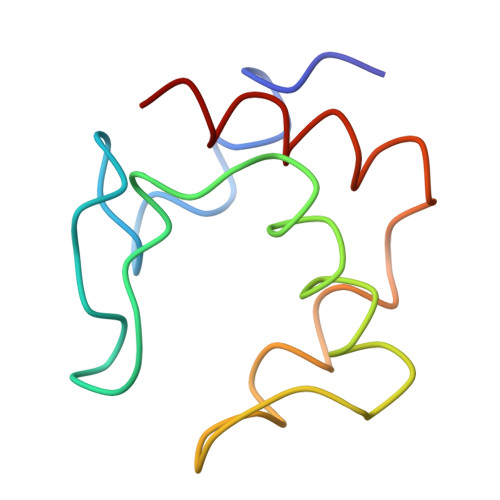
S-class cytochromes c have a variety of folding patterns: structure of cytochrome c-553 from Desulfovibrio vulgaris determined by the multi-wavelength anomalous dispersion method.
Nakagawa, A., Higuchi, Y., Yasuoka, N., Katsube, Y., Yagi, T.(1990) J Biochem 108: 701-703
- PubMed: 1964450
- DOI: https://doi.org/10.1093/oxfordjournals.jbchem.a123267
- Primary Citation of Related Structures:
1C53 - PubMed Abstract:
The three-dimensional structure of cytochrome c-553 isolated from sulfate-reducing bacterium, Desulfovibrio vulgaris Miyazaki F strain, has been determined by the multi-wavelength anomalous dispersion technique with use of synchrotron radiation. The result shows that bacterial S-class cytochromes c have a variety of folding patterns. The relative location of two a-helices at amino- and carboxyl-terminals and the style of bonding to the heme group show "cytochrome c folding," but other regions of the structure are different from those of other cytochromes c previously reported. The results also give useful information about the location of sulfate-reducing bacterium on the phylogenetic tree of the bacterial cytochromes c superfamily.
Organizational Affiliation:
Photon Factory, National Laboratory for High Energy Physics, Hyogo.