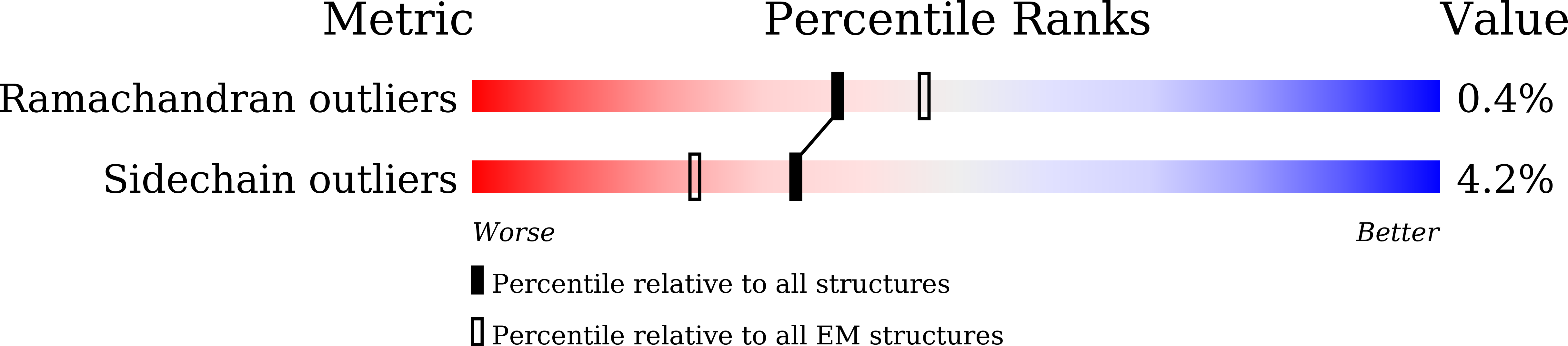Structural morphing in the viral portal vertex of bacteriophage lambda.
Gu, Z., Wu, K., Wang, J.(2024) J Virol : e0006824-e0006824
- PubMed: 38661364
- DOI: https://doi.org/10.1128/jvi.00068-24
- Primary Citation of Related Structures:
8XOT, 8XOU, 8XOW, 8XPM, 8XQB - PubMed Abstract:
The portal protein of tailed bacteriophage plays essential roles in various aspects of capsid assembly, motor assembly, genome packaging, connector formation, and infection processes. After DNA packaging is complete, additional proteins are assembled onto the portal to form the connector complex, which is crucial as it bridges the mature head and tail. In this study, we report high-resolution cryo-electron microscopy (cryo-EM) structures of the portal vertex from bacteriophage lambda in both its prohead and mature virion states. Comparison of these structures shows that during head maturation, in addition to capsid expansion, the portal protein undergoes conformational changes to establish interactions with the connector proteins. Additionally, the independently assembled tail undergoes morphological alterations at its proximal end, facilitating its connection to the head-tail joining protein and resulting in the formation of a stable portal-connector-tail complex. The B-DNA molecule spirally glides through the tube, interacting with the nozzle blade region of the middle-ring connector protein. These insights elucidate a mechanism for portal maturation and DNA translocation within the phage lambda system. The tailed bacteriophages possess a distinct portal vertex that consists of a ring of 12 portal proteins associated with a 5-fold capsid shell. This portal protein is crucial in multiple stages of virus assembly and infection. Our research focused on examining the structures of the portal vertex in both its preliminary prohead state and the fully mature virion state of bacteriophage lambda. By analyzing these structures, we were able to understand how the portal protein undergoes conformational changes during maturation, the mechanism by which it prevents DNA from escaping, and the process of DNA spirally gliding.
Organizational Affiliation:
State Key Laboratory of Membrane Biology, Beijing Frontier Research Center for Biological Structure, School of Life Sciences, Tsinghua University, Beijing, China.