Immunobiological properties and structure analysis of group 13 allergen from Blomia tropicalis and its IgE-mediated cross-reactivity.
Zhou, Y., Zhu, K., Li, Q., Zhou, D., Ren, Y., Liao, Y., Cao, P., Gong, Y., Cui, Y.(2023) Int J Biol Macromol 254: 127788-127788
- PubMed: 37926306
- DOI: https://doi.org/10.1016/j.ijbiomac.2023.127788
- Primary Citation of Related Structures:
8WKH - PubMed Abstract:
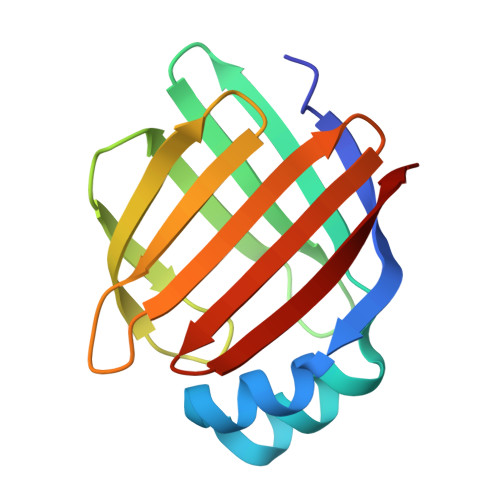
Blomia tropicalis is an important species of allergenic mite. Structurally related cross-reactive allergens are involved in pathogenesis of clinical symptoms. The present study focused on recombinant allergen rBlo t 13 from B. tropicalis, including investigation of its structure, immunological properties, IgE-mediated cross-reactivity. In this work, the prokaryotic expression plasmids pET-28(a)-Blo t 13, pET-28(a)-Der f 13, and pET-28(a)-Tyr p 13 were constructed, transformed into E. coli Rosetta (DE3) pLysS, and purified by nickel affinity chromatography, respectively. By using ELISA, the IgE-binding rates were detected for rBlo t 13 and its epitope peptides, as well as the cross-reactivity among rBlo t 13, rDer f 13, and rTyr p 13. The tertiary structure of rBlo t 13 was resolved using X-ray diffraction at 2.0 Å resolution. Using IgE-ELISA, the IgE binding rate of rBlo t 13 was 60 % with Blomia tropicalis-positive sera. In the experiments of ELISA for cross-reactivity with rBlo t 13 on solid phase, the inhibition rates were 65 %, 57 % and 63 % for rBlo t 13, rDer f 13, and rTyr p 13, respectively. The structure of Blo t 13 protein contains a β-barrel structure which is composed of 10 β strands and has 2 α helices at the end of the barrel. Comparison of the tertiary structures of rBlo t 13, rDer f 13, and rTyr p 13 revealed that the β-barrel structure is highly conserved, consistent with the alignment of amino acid sequences. We obtained the recombinant protein rBlo t 13, demonstrated its cross-reactivity with Der f 13 and Tyr p 13 due to their structural similarity.
Organizational Affiliation:
Department of Pediatrics Laboratory, The Affiliated Wuxi Children's Hospital of Jiangnan University, Wuxi 214023, PR China.