Autoinhibition and activation of myosin VI revealed by its cryo-EM structure.
Niu, F., Li, L., Wang, L., Xiao, J., Xu, S., Liu, Y., Lin, L., Yu, C., Wei, Z.(2024) Nat Commun 15: 1187-1187
- PubMed: 38331992
- DOI: https://doi.org/10.1038/s41467-024-45424-7
- Primary Citation of Related Structures:
8W41 - PubMed Abstract:
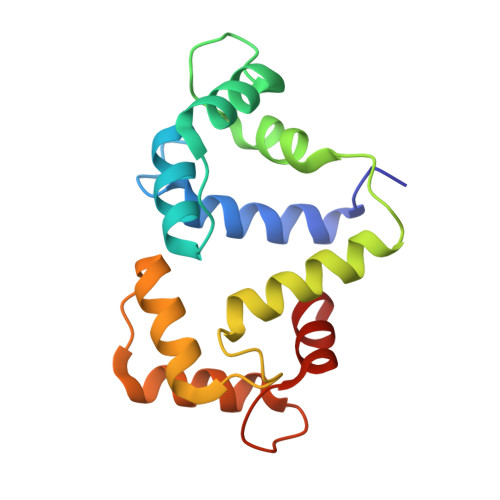
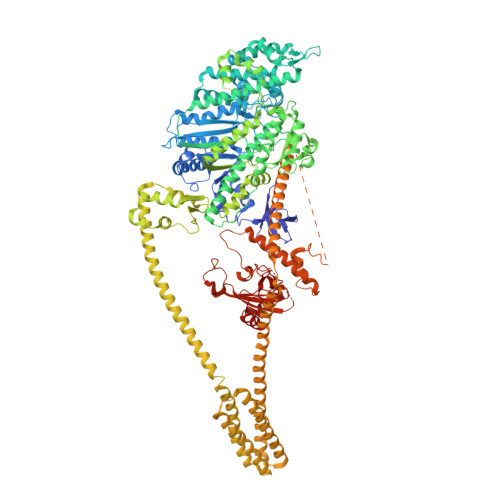
Myosin VI is the only molecular motor that moves towards the minus end along actin filaments. Numerous cellular processes require myosin VI and tight regulations of the motor's activity. Defects in myosin VI activity are known to cause genetic diseases such as deafness and cardiomyopathy. However, the molecular mechanisms underlying the activity regulation of myosin VI remain elusive. Here, we determined the high-resolution cryo-electron microscopic structure of myosin VI in its autoinhibited state. Our structure reveals that autoinhibited myosin VI adopts a compact, monomeric conformation via extensive interactions between the head and tail domains, orchestrated by an elongated single-α-helix region resembling a "spine". This autoinhibited structure effectively blocks cargo binding sites and represses the motor's ATPase activity. Certain cargo adaptors such as GIPC can release multiple inhibitory interactions and promote motor activity, pointing to a cargo-mediated activation of the processive motor. Moreover, our structural findings allow rationalization of disease-associated mutations in myosin VI. Beyond the activity regulation mechanisms of myosin VI, our study also sheds lights on how activities of other myosin motors such as myosin VII and X might be regulated.
Organizational Affiliation:
Department of Neuroscience and Brain Research Center, School of Life Sciences, Southern University of Science and Technology, Shenzhen, Guangdong, China.