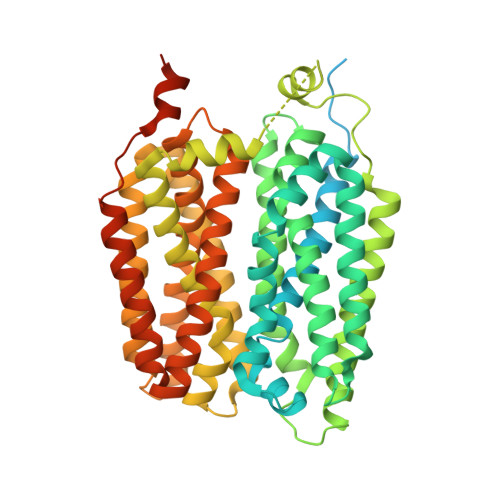
Structural and molecular basis of choline uptake into the brain by FLVCR2.
Cater, R.J., Mukherjee, D., Gil-Iturbe, E., Erramilli, S.K., Chen, T., Koo, K., Santander, N., Reckers, A., Kloss, B., Gawda, T., Choy, B.C., Zhang, Z., Katewa, A., Larpthaveesarp, A., Huang, E.J., Mooney, S.W.J., Clarke, O.B., Yee, S.W., Giacomini, K.M., Kossiakoff, A.A., Quick, M., Arnold, T., Mancia, F.(2024) Nature 629: 704-709
- PubMed: 38693257
- DOI: https://doi.org/10.1038/s41586-024-07326-y
- Primary Citation of Related Structures:
8VZN, 8VZO - PubMed Abstract:
Choline is an essential nutrient that the human body needs in vast quantities for cell membrane synthesis, epigenetic modification and neurotransmission. The brain has a particularly high demand for choline, but how it enters the brain remains unknown 1-3 . The major facilitator superfamily transporter FLVCR1 (also known as MFSD7B or SLC49A1) was recently determined to be a choline transporter but is not highly expressed at the blood-brain barrier, whereas the related protein FLVCR2 (also known as MFSD7C or SLC49A2) is expressed in endothelial cells at the blood-brain barrier 4-7 . Previous studies have shown that mutations in human Flvcr2 cause cerebral vascular abnormalities, hydrocephalus and embryonic lethality, but the physiological role of FLVCR2 is unknown 4,5 . Here we demonstrate both in vivo and in vitro that FLVCR2 is a BBB choline transporter and is responsible for the majority of choline uptake into the brain. We also determine the structures of choline-bound FLVCR2 in both inward-facing and outward-facing states using cryo-electron microscopy. These results reveal how the brain obtains choline and provide molecular-level insights into how FLVCR2 binds choline in an aromatic cage and mediates its uptake. Our work could provide a novel framework for the targeted delivery of therapeutic agents into the brain.
Organizational Affiliation:
Department of Physiology and Cellular Biophysics, Columbia University, New York, NY, USA. r.cater@uq.edu.au.