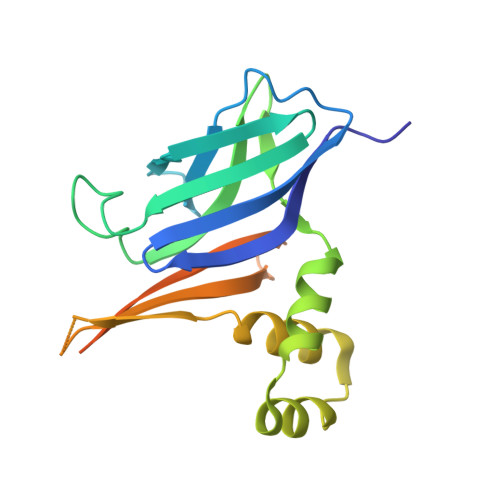
Cryo-EM analysis of S. aureus TarL, a polymerase in wall teichoic acid biogenesis central to virulence and antibiotic resistance.
Li, F.K.K., Worrall, L.J., Gale, R.T., Brown, E.D., Strynadka, N.C.J.(2024) Sci Adv 10: eadj3864-eadj3864
- PubMed: 38416829
- DOI: https://doi.org/10.1126/sciadv.adj3864
- Primary Citation of Related Structures:
8V33, 8V34, 8VA1 - PubMed Abstract:
Wall teichoic acid (WTA), a covalent adduct of Gram-positive bacterial cell wall peptidoglycan, contributes directly to virulence and antibiotic resistance in pathogenic species. Polymerization of the Staphylococcus aureus WTA ribitol-phosphate chain is catalyzed by TarL, a member of the largely uncharacterized TagF-like family of membrane-associated enzymes. We report the cryo-electron microscopy structure of TarL, showing a tetramer that forms an extensive membrane-binding platform of monotopic helices. TarL is composed of an amino-terminal immunoglobulin-like domain and a carboxyl-terminal glycosyltransferase-B domain for ribitol-phosphate polymerization. The active site of the latter is complexed to donor substrate cytidine diphosphate-ribitol, providing mechanistic insights into the catalyzed phosphotransfer reaction. Furthermore, the active site is surrounded by electropositive residues that serve to retain the lipid-linked acceptor for polymerization. Our data advance general insight into the architecture and membrane association of the still poorly characterized monotopic membrane protein class and present molecular details of ribitol-phosphate polymerization that may aid in the design of new antimicrobials.
Organizational Affiliation:
Department of Biochemistry and Molecular Biology, The University of British Columbia, Vancouver, British Columbia, Canada.