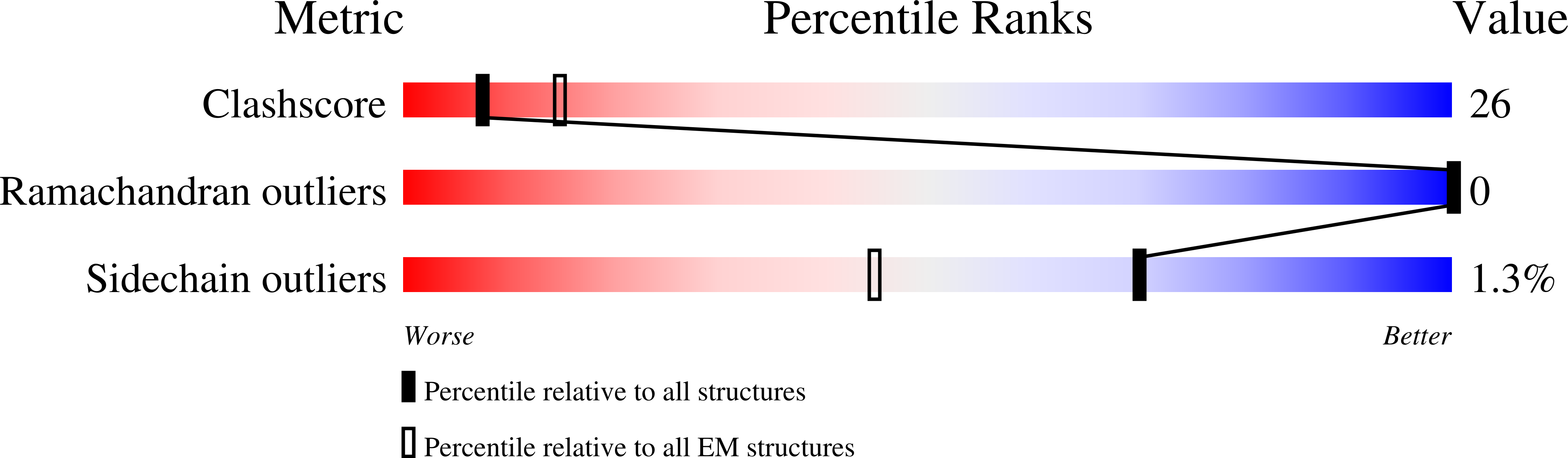Structure of the Drosophila melanogaster Flight Muscle Myosin Filament at 4.7 angstrom Resolution Reveals New Details of Non-Myosin Proteins.
Abbasi Yeganeh, F., Rastegarpouyani, H., Li, J., Taylor, K.A.(2023) Int J Mol Sci 24
- PubMed: 37834384
- DOI: https://doi.org/10.3390/ijms241914936
- Primary Citation of Related Structures:
8U8H, 8U95 - PubMed Abstract:
Striated muscle thick filaments are composed of myosin II and several non-myosin proteins which define the filament length and modify its function. Myosin II has a globular N-terminal motor domain comprising its catalytic and actin-binding activities and a long α-helical, coiled tail that forms the dense filament backbone. Myosin alone polymerizes into filaments of irregular length, but striated muscle thick filaments have defined lengths that, with thin filaments, define the sarcomere structure. The motor domain structure and function are well understood, but the myosin filament backbone is not. Here we report on the structure of the flight muscle thick filaments from Drosophila melanogaster at 4.7 Å resolution, which eliminates previous ambiguities in non-myosin densities. The full proximal S2 region is resolved, as are the connecting densities between the Ig domains of stretchin-klp. The proteins, flightin, and myofilin are resolved in sufficient detail to build an atomic model based on an AlphaFold prediction. Our results suggest a method by which flightin and myofilin cooperate to define the structure of the thick filament and explains a key myosin mutation that affects flightin incorporation. Drosophila is a genetic model organism for which our results can define strategies for functional testing.
Organizational Affiliation:
Institute of Molecular Biophysics, Florida State University, Tallahassee, FL 32306-4380, USA.